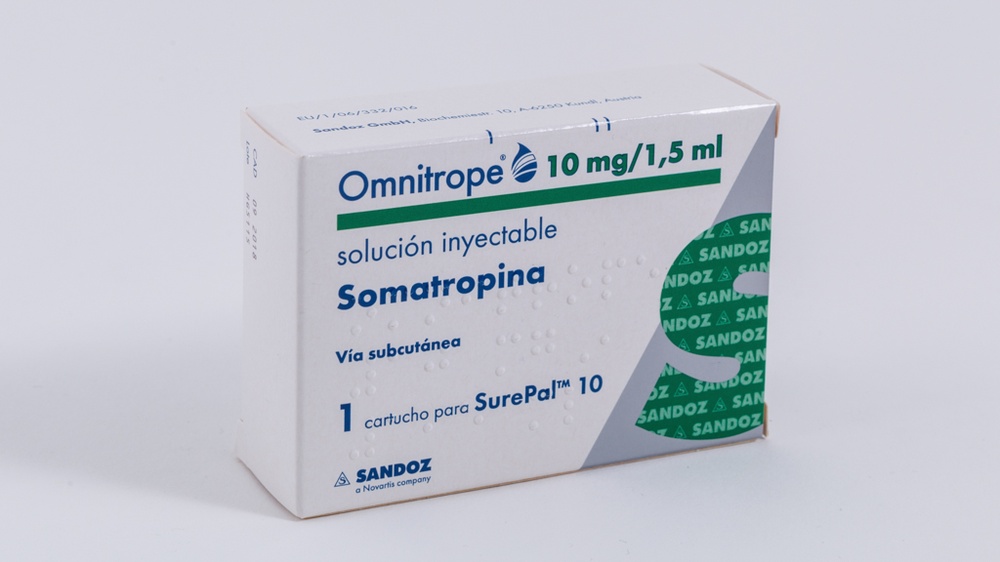
OMNITROPE 10 MG/1,5 ML SOLUCION INYECTABLE EN CARTUCHO

Cómo usar OMNITROPE 10 MG/1,5 ML SOLUCION INYECTABLE EN CARTUCHO
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Omnitrope 5mg/1,5ml solución inyectable en cartucho
Omnitrope 10mg/1,5ml solución inyectable en cartucho
Omnitrope 15mg/1,5ml solución inyectable en cartucho
somatropina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Omnitrope y para qué se utiliza
- Qué necesita saber antes de empezar a usar Omnitrope
- Cómo usar Omnitrope
- Posibles efectos adversos
- Conservación de Omnitrope
- Contenido del envase e información adicional
1. Qué es Omnitrope y para qué se utiliza
Omnitrope es una hormona del crecimiento humana recombinante (también llamada somatropina). Tiene la misma estructura que la hormona del crecimiento humana natural, que es necesaria para que los huesos y los músculos crezcan. También ayuda a que los tejidos grasos y musculares se desarrollen en las cantidades correctas. Es recombinante, lo que significa que no se elabora a partir de tejido humano o animal.
En los niños, Omnitrope se usa para tratar los siguientes trastornos del crecimiento:
- Si no creces adecuadamente y no tienes suficiente hormona del crecimiento propia.
- Si padeces un síndrome de Turner, que es un trastorno genético en las niñas que puede afectar al crecimiento; el médico te lo habrá dicho si padeces este trastorno.
- Si padeces una insuficiencia renal crónica. A medida que los riñones pierden su capacidad para funcionar normalmente, esto puede afectar al crecimiento.
- Si eras demasiado pequeño o menudo al nacer. La hormona de crecimiento puede ayudar a que crezcas más si no has podido tener un estirón o mantener un crecimiento normal a los cuatro años de edad o en adelante.
- Si padeces un síndrome de Prader-Willi (un trastorno cromosómico). La hormona de crecimiento puede ayudar a que crezca más si sigue creciendo y también mejorará la composición de tu organismo. El exceso de grasa se reducirá y la masa muscular disminuida mejorará.
En los adultos, Omnitrope se usa para
- tratar a las personas con una deficiencia pronunciada de la hormona del crecimiento. Esta puede empezar durante la edad adulta o puede continuar desde la niñez.
Si usted ha sido tratado con Omnitrope por una deficiencia de la hormona de crecimiento durante la niñez, se volverá a examinar el estado de la hormona de crecimiento después de finalizar el crecimiento. Si se confirma una deficiencia grave de la hormona, el médico propondrá la continuación del tratamiento con Omnitrope.
Solo debe recibir este medicamento de un médico que tenga experiencia con la hormona de crecimiento y que haya confirmado su diagnóstico.
2. Qué necesita saber antes de empezar a usar Omnitrope
No use Omnitrope
- si es alérgico (hipersensible) a somatropina o a cualquiera de los demás componentes de Omnitrope.
- e informe a su médico si usted padece un tumor activo (cáncer). Los tumores deben ser inactivos y usted debe haber terminado su tratamiento antitumoral antes de empezar su tratamiento con Omnitrope.
- e informe a su médico si se le ha prescrito Omitrope para estimular el crecimiento pero usted ya ha dejado de crecer (epífisis cerradas).
- si está gravemente enfermo (por ejemplo, complicaciones pos-quirúrgicas a corazón abierto, cirugía abdominal, traumatismo accidental, insuficiencia respiratoria aguda o afecciones similares). Si a usted le van a practicar o le han practicado una operación mayor, o si va al hospital por cualquier motivo, infórmele a su médico y recuérdeles a los otros médicos a los que ve que usted usa la hormona de crecimiento.
Advertencias y precauciones
Consulte a su médico antes de empezar a usar Omnitrope.
- Si está recibiendo una terapia de sustitución con glucocorticoides, debe consultar con su médico regularmente ya que puede ser necesario un ajuste de su dosis de glucocorticoide.
- Si tiene riesgo de presentar diabetes, el médico deberá controlar regularmente la concentración de la glucosa en la sangre durante el tratamiento con somatropina.
- Si padece diabetes mellitus, deberá vigilar atentamente la concentración de glucosa en la sangre durante el tratamiento con somatropina y hablar con el médico acerca de los resultados, a fin de decidir si tiene que cambiar la dosis de sus medicamentos para tratar la diabetes.
- Después de comenzar el tratamiento con somatropina, algunos pacientes pueden tener que comenzar un reemplazo con hormona tiroidea.
- Si recibe tratamiento con hormonas tiroideas, puede ser necesario ajustar la dosis de hormona tiroidea.
- Si usted tiene un aumento de la presión intracraneal (que causa síntomas, tales como dolor de cabeza intenso, alteraciones visuales o vómitos) deberá informar al médico acerca de ello.
- Si camina cojeando o si empieza a cojear durante el tratamiento con hormona de crecimiento, deberá informar al médico.
- Si está recibiendo somatropina para una deficiencia de la hormona de crecimiento después de un tumor previo (cáncer), deberán examinarlo regularmente para detectar la recurrencia del tumor o cualquier otro cáncer.
- Si experimenta un dolor abdominal que empeora, debe informar a su médico.
- La experiencia en pacientes de más de 80 años es limitada. Las personas de edad avanzada pueden ser más sensibles a la acción de somatropina y, por lo tanto, pueden ser más propensas a presentar reacciones adversas.
- Omnitrope puede causar una inflamación del páncreas, que provoca dolor intenso en el abdomen y la espalda. Póngase en contacto con su médico si usted o su hijo/a presentan dolor de estómago después de la administración de Omnitrope.
- La curvatura lateral de la columna (escoliosis) puede aumentar en cualquier niño durante el crecimiento rápido. El médico le examinará a usted (o a su hijo/a) para detectar signos de escoliosis durante el tratamiento con somatropina.
Niños con insuficiencia renal crónica
- El médico deberá examinar la función de los riñones y la velocidad de crecimiento antes de empezar el tratamiento con somatropina. El tratamiento médico de los riñones debe continuarse. El tratamiento con somatropina debe interrumpirse en caso de trasplante renal.
Niños con síndrome de Prader-Willi
- El médico le dará restricciones en la dieta que debe seguir para controlar su peso.
- El médico evaluará los signos de obstrucción de las vías respiratorias altas, apnea del sueño (en que la respiración se interrumpe durante el sueño) o infección respiratoria antes de comenzar el tratamiento con somatropina.
- Durante el tratamiento con somatropina, informe al médico si presenta signos de obstrucción de las vías respiratorias altas (lo que incluye comenzar a roncar o un empeoramiento de los ronquidos). Tal vez el médico tenga que examinarle y puede interrumpir el tratamiento con somatropina.
- Durante el tratamiento, el médico le examinará para ver si hay signos de escoliosis, un tipo de deformidad vertebral.
- Durante el tratamiento, si presenta una infección pulmonar, informe al médico para que pueda tratar la infección.
Niños nacidos demasiado pequeños o bajos de peso
- Si eras demasiado pequeño o menudo al nacer y tienes de 9 a 12 años, consulta al médico específicamente en relación con la pubertad y el tratamiento con este medicamento.
- El tratamiento debe continuar hasta que hayas dejado de crecer.
- El médico examinará las concentraciones de glucosa e insulina antes de comenzar el tratamiento y cada año durante el tratamiento.
Uso de Omnitrope con otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
En particular, informe a su médico si está tomando o recientemente ha tomado alguno de los siguientes medicamentos. Puede que su médico necesite ajustar la dosis de Omnitrope o de los otros medicamentos:
- medicamentos para tratar la diabetes;
- hormonas tiroideas;
- medicamentos para controlar la epilepsia (anticonvulsivantes);
- ciclosporina (un medicamento que debilita el sistema inmunitario después de los trasplantes);
- estrógenos administrados por vía oral u otras hormonas sexuales;
- hormonas suprarrenales sintéticas (corticoesteroides).
Tal vez el médico tenga que ajustar la dosis de estos medicamentos o la dosis de somatropina
Embarazo y lactancia
No debe usar Omnitrope si está embarazada o tratando de quedarse embarazada.
Consulte a su médico o farmacéutico si está embarazada o en período de lactancia. El motivo es que puede formarse alcohol bencílico en su organismo y provocar efectos secundarios (llamados “acidosis metabólica”).
Información importante sobre algunos de los componentes de Omnitrope
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por ml; esto es, esencialmente “exento de sodio”.
Omnitrope 5 mg/1,5 ml solución inyectable:
Este medicamento contiene 9 mg de alcohol bencílico en cada ml. El alcohol bencílico puede provocar reacciones alérgicas.
El alcohol bencílico se ha relacionado con el riesgo de efectos adversos graves, incluidos problemas respiratorios (llamados “síndrome de jadeo”) en niños pequeños.
No debe administrárselo a su hijo recién nacido (de hasta 4 semanas de edad), salvo que se lo recomiende su médico.
Si tiene una enfermedad hepática o renal, consulte a su médico o farmacéutico. El motivo es que podrían formarse en su organismo grandes cantidades de alcohol bencílico y provocar efectos secundarios (llamados “acidosis metabólica”).
Debido a la presencia de alcohol bencílico, el medicamento no se debe administrar a niños prematuros ni recién nacidos. Puede provocar reacciones tóxicas y reacciones alérgicas en niños de hasta 3 años de edad.
No lo use durante más de una semana en niños pequeños (de menos de 3 años de edad), a menos que se lo aconseje su médico o farmacéutico.
3. Cómo usar Omnitrope
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico, farmacéutico o enfermero. En caso de duda, consulte de nuevo a su médico, farmacéutico o enfermero.
La dosis depende de su tamaño, de la afección para la que recibe tratamiento y de lo bien que funcione la hormona de crecimiento en usted. Todas las personas son diferentes. El médico le aconsejará acerca de su dosis individualizada de Omnitrope en miligramos (mg) a partir de su peso corporal en kilogramos (kg) o por su superficie corporal, calculada a partir de su estatura y peso en metros cuadrados (m2), así como su pauta de tratamiento. No cambie la dosificación y la pauta de tratamiento sin consultarle al médico.
La dosis recomendada es para:
Niños con deficiencia de la hormona de crecimiento:
0,025 a 0,035 mg/kg de peso corporal al día o 0,7 a 1,0 mg/m2 de superficie corporal al día. Pueden utilizarse dosis más altas. Cuando la deficiencia de hormona de crecimiento continúa durante la adolescencia, Omnitrope debe continuarse hasta finalizar el desarrollo físico.
Niñas con síndrome de Turner:
0,045 a 0,050 mg/kg de peso corporal al día o 1,4 mg/m2 de superficie corporal al día.
Niños con insuficiencia renal crónica:
0,045 a 0,050 mg/kg de peso corporal al día o 1,4 mg/m2 de superficie corporal al día. Pueden ser necesarias dosis más altas si la velocidad de crecimiento es demasiado baja. Puede ser necesario un ajuste de la dosis después de seis meses de tratamiento.
Niños con síndrome de Prader-Willi:
0,035 mg/kg de peso corporal al día o 1,0 mg/m2 de superficie corporal al día. La dosificación diaria no debe ser superior a 2,7 mg. El tratamiento no debe utilizarse en los niños que casi han dejado de crecer después de la pubertad.
Niños nacidos más pequeños o con peso más bajo que lo esperado y con un trastorno del crecimiento:
0,035 mg/kg de peso corporal al día o 1,0 mg/m2 de superficie corporal al día. Es importante continuar el tratamiento hasta que se alcance la estatura final. El tratamiento debe suspenderse después del primer año si no responde, o si ha alcanzado la estatura final y dejado de crecer.
Adultos con deficiencia de la hormona de crecimiento:
Si continúa utilizando Omnitrope después del tratamiento durante la niñez, debe comenzar con 0,2 a 0,5 mg al día.
Esta dosificación se debe aumentar o reducir gradualmente según los resultados de los análisis de sangre, así como la respuesta clínica y los efectos secundarios.
Si la deficiencia de la hormona de crecimiento comienza durante la vida adulta, debe comenzar con 0,15 a 0,3 mg al día. Esta dosificación debe aumentarse gradualmente según los resultados de los análisis de sangre, así como la respuesta clínica y los efectos secundarios. La dosis de mantenimiento diaria rara vez es superior a 1,0 mg diarios. Las mujeres pueden necesitar dosis más altas que los hombres. La dosificación debe vigilarse cada seis meses. Las personas de más de 60 años deben comenzar con una dosis de 0,1 a 0,2 mg diarios que debe aumentarse lentamente según las necesidades individuales. Debe utilizarse la dosis mínima eficaz. La dosis de mantenimiento rara vez supera 0,5 mg al día. Siga las instrucciones que le haya dado el médico.
Inyección de Omnitrope
Inyéctese la hormona del crecimiento más o menos a la misma hora cada día. La hora de acostarse es un buen momento porque es fácil de recordar. Además, también es natural tener una concentración más elevada de hormona del crecimiento por la noche.
Omnitrope 5 mg/1,5 ml en un cartucho para SurePal 5 está pensado para varios usos. Solo debe administrarse con SurePal 5, un dispositivo inyectable desarrollado específicamente para su uso con Omnitrope 5 mg/1,5 ml solución inyectable.
Omnitrope 10 mg/1,5 ml en un cartucho para SurePal 10 está pensado para varios usos. Solo debe administrarse con SurePal 10, un dispositivo inyectable desarrollado específicamente para su uso con Omnitrope 10 mg/1,5 ml solución inyectable.
Omnitrope 15 mg/1,5 ml en un cartucho para SurePal 15 está pensado para varios usos. Solo debe administrarse con SurePal 15, un dispositivo inyectable desarrollado específicamente para su uso con Omnitrope 15 mg/1,5 ml solución inyectable.
Omnitrope está indicado para su empleo por vía subcutánea. Esto significa que se inyecta por medio de una pequeña aguja para inyección en el tejido adiposo, por debajo de la piel. La mayor parte de las personas se inyectan en el muslo o en las nalgas. Póngase la inyección en el sitio que le haya enseñado su médico. El tejido adiposo de la piel puede verse reducido en el lugar de la inyección. Para evitar esto, utilice cada vez un sitio ligeramente diferente para inyectarse. Esto proporciona a la piel y a la zona por debajo de ella tiempo para recuperarse de una inyección antes de recibir otra en el mismo sitio.
El médico debe haberle enseñado ya cómo utilizar Omnitrope. Inyéctese siempre Omnitrope tal como el médico le ha dicho. Si no está seguro, compruebe con su médico o farmacéutico.
Cómo inyectar Omnitrope
Las siguientes instrucciones explican cómo inyectarse Omnitrope usted mismo. Lea detenidamente las instrucciones y sígalas paso a paso. Su médico o la enfermera le enseñará cómo inyectarse Omnitrope. No intente inyectarse a menos que esté seguro de que entiende el procedimiento y lo que conlleva la inyección.
- Omnitrope se administra como una inyección debajo de la piel.
- Inspeccione cuidadosamente la solución antes de inyectarla y úsela solo si es clara e incolora.
- Cambie el lugar de la inyección para así minimizar el riesgo de lipoatrofia local (reducción local del tejido adiposo debajo de la piel).
Preparación | |
Antes de empezar, debe tener todo lo necesario: |
|
| |
| |
| |
| |
Lávese las manos antes de continuar con los siguiente pasos. | |
Inyección de Omnitrope | |
|
|
| |
| |
| |
|
|
| |
Después de inyectarse | |
| |
| |
| |
|
Si usa más Omnitrope del que debe
Si se inyecta mucho más de lo que debiera, consulte lo antes posible a su médico o farmacéutico. Su concentración de azúcar en la sangre podría descender demasiado y después aumentar demasiado. Tal vez se sienta con temblores, sudoroso, somnoliento o “como si no fuera usted mismo”, y podría desmayarse.
Si olvidó usar Omnitrope
No use una dosis doble para compensar las dosis olvidadas. Lo mejor es usar la hormona de crecimiento con regularidad. Si se olvida de usar una dosis, póngase la siguiente inyección a la hora habitual, al día siguiente. Tome nota de las inyecciones olvidadas e infórmele al médico en el siguiente control.
Si interrumpe el tratamiento con Omnitrope
Consulte a su médico antes de dejar de usar Omnitrope.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran. Los efectos adversos muy frecuentes y frecuentes en los adultos pueden comenzar en los primeros meses de tratamiento y pueden detenerse espontáneamente o si se reduce la dosis.
Los efectos adversos muy frecuentes (que probablemente se produzcan en más de 1 de cada 10pacientes) incluyen los siguientes:
- Dolor en las articulaciones
- Retención de agua (que se presenta como hinchazón de los dedos o los tobillos, durante un breve periodo de tiempo al inicio del tratamiento)
- Enrojecimiento, picor o dolor en el lugar de la inyección)
Los efectos adversos frecuentes (que probablemente afecten a menos de 1 de cada 10pacientes) incluyen los siguientes:
- Ronchas pruriginosas en la piel
- Erupción cutánea
- Adormecimiento, hormigueos
- Rigidez de los brazos y las piernas, dolor muscular
En los adultos
- Dolor o sensación de escozor en las manos o los antebrazos (conocido como síndrome del túnel carpiano)
Los efectos adversos poco frecuentes (que probablemente afecten a menos de 1 de cada 100pacientes) incluyen los siguientes:
- Aumento del tamaño de las mamas (ginecomastia)
- Comezón
Los efectos adversos raros (que probablemente afecten a menos de 1 de cada 1.000pacientes) incluyen los siguientes:
En los niños
- Leucemia (se ha observado en un pequeño número de pacientes con deficiencia de la hormona del crecimiento, algunos de cuales habían sido tratados con somatropina. Sin embargo, no hay ningún indicio de que la incidencia de leucemia sea mayor en receptores de la hormona del crecimiento sin factores predisponentes)
- Aumento de la presión intracraneal (que causa síntomas, como cefalea intensa, alteraciones visuales o vómitos)
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles)
- Diabetes de tipo 2
- Disminución de los niveles de la hormona cortisol en la sangre
- Hinchazón facial
- Dolor de cabeza
- Hipotiroidismo
En los adultos
- Aumento de la presión intracraneal (que causa síntomas, como dolor de cabeza intenso, alteraciones visuales o vómitos)
Formación de anticuerpos contra la hormona del crecimiento inyectada, pero estos no parecen hacer que la hormona del crecimiento deje de funcionar.
La piel alrededor de la zona de inyección puede ponerse irregular o con bultos, pero esto no deberá ocurrir si se inyecta en un lugar diferente cada vez.
Ha habido casos raros de muerte súbita en pacientes con síndrome de Prader-Willi. No obstante, estos casos no se han relacionado con el tratamiento con Omnitrope.
Su médico puede considerar una epifisiólisis de la cabeza femoral o una enfermedad de Legg-Calvé-Perthes si experimenta molestias o dolor en la cadera o la rodilla mientras está siendo tratado con Omnitrope.
Otros posibles efectos adversos relacionados con su tratamiento con la hormona del crecimiento pueden incluir los siguientes:
Usted (o su hijo) puede tener niveles elevados de azúcar en sangre o niveles reducidos de la hormona tiroidea. Esto lo puede analizar su médico y, si es necesario, su médico le recetará el tratamiento adecuado. En casos raros se ha observado inflamación del páncreas en pacientes tratados con la hormona del crecimiento.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Omnitrope
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y en la caja después de CAD. La fecha de caducidad es el último día del mes que se indica.
- Conservar y transportar refrigerado (entre 2°C y 8°C).
- No congelar.
- Conservar en el embalaje original para protegerlo de la luz.
- Después de la administración de la primera inyección, el cartucho debe permanecer en el inyector de pluma y debe conservarse en una nevera, a una temperatura de 2 a 8 ºC, y solo debe usarse durante un máximo de 28 días.
No utilice Omnitrope si observa que la solución está turbia.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Omnitrope 5mg/1,5ml
- El principio activo de Omnitrope es somatropina.
Cada ml de solución contiene 3,3 mg de somatropina (que corresponde a 10 UI).
Un cartucho contiene 5,0 mg (que corresponde a 15 UI) de somatropina en 1,5 ml.
- Los demás componentes son:
Fosfato hidrógeno disódico heptahidrato
Fosfato dihidrógeno sódico dihidrato
Manitol
Poloxámero 188
Alcohol bencílico
Agua para preparaciones inyectables
Composición de Omnitrope 10mg/1,5ml
- El principio activo de Omnitrope es somatropina.
Cada ml de solución contiene 6,7 mg de somatropina (que corresponde a 20 UI).
Un cartucho contiene 10,0 mg (que corresponden a 30 UI) de somatropina en 1,5 ml.
- Los demás componentes son:
Fosfato hidrógeno disódico heptahidrato
Fosfato dihidrógeno sódico dihidrato
Glicina
Poloxámero 188
Fenol
Agua para preparaciones inyectables
Composición de Omnitrope 15mg/1,5ml
- El principio activo de Omnitrope es somatropina.
Cada ml de solución contiene 10 mg de somatropina (que corresponde a 30 UI).
Un cartucho contiene 15,0 mg (que corresponden a 45 UI) de somatropina en 1,5 ml.
- Los demás componentes son:
Fosfato hidrógeno disódico heptahidrato
Fosfato dihidrógeno sódico dihidrato
Cloruro de sodio
Poloxámero 188
Fenol
Agua para preparaciones inyectables
Aspecto del producto y contenido del envase
Omnitrope es una solución inyectable transparente e incolora.
Omnitrope 5 mg/1,5 ml solución para inyección es para su uso solo en SurePal 5.
Omnitrope 10 mg/1,5 ml solución para inyección es para su uso solo en SurePal 10.
Omnitrope 15 mg/1,5 ml solución para inyección es para su uso solo en SurePal 15.
Envases con 1, 5 o 10 cartuchos.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Sandoz GmbH
Biochemiestr. 10
A-6250 Kundl
Austria
Responsable de la fabricación
Sandoz GmbH
Biochemiestr. 10
A-6336 Langkampfen
Austria
Novartis Pharmaceutical Manufacturing GmbH
Biochemiestr. 10
A-6336 Langkampfen
Austria
Fecha de la última revisión de este prospecto: {MM/AAAA} .
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Sandoz nv/sa Tél/Tel: +32 2 722 97 97 | Lietuva Sandoz Pharmaceuticals d.d filialas Tel: +370 5 2636 037 |
???????? ?????? ???????? ??? ???.: +359 2 970 47 47 | Luxembourg/Luxemburg Sandoz nv/sa (Belgique/Belgien) Tél/Tel.: +32 2 722 97 97 |
Ceská republika Sandoz s.r.o. Tel: +420 225 775 111 | Magyarország Sandoz Hungária Kft. Tel.: +36 1 430 2890 |
Danmark/Norge/Ísland/Sverige Sandoz A/S Tlf: +45 63 95 10 00 | Malta Sandoz Pharmaceuticals d.d. Tel: +35699644126 |
Deutschland Hexal AG Tel: +49 8024 908 0 | Nederland Sandoz B.V. Tel: +31 36 52 41 600 |
Eesti Sandoz d.d. Eesti filiaal Tel: +372 665 2400 | Österreich Sandoz GmbH Tel: +43 5338 2000 |
Ελλ?δα SANDOZ HELLAS ΜΟΝΟΠΡΟΣΩΠΗ Α.Ε. Τηλ: +30 216 600 5000 | Polska Sandoz Polska Sp. z o.o. Tel.: +48 22 209 70 00 |
España Sandoz Farmacéutica, S.A. Tel: +34 900 456 856 | Portugal Sandoz Farmacêutica Lda. Tel: +351 21 000 86 00 |
France Sandoz SAS Tél: +33 1 49 64 48 00 | România Sandoz Pharmaceuticals SRL Tel: +40 21 407 51 60 |
Hrvatska Sandoz d.o.o. Tel: +385 1 23 53 111 | Slovenija Sandoz farmacevtska družba d.d. Tel: +386 1 580 29 02 |
Ireland Rowex Ltd. Tel: + 353 27 50077 | Slovenská republika Sandoz d.d. - organizacná zložka Tel: +421 2 48 200 600 |
Italia Sandoz S.p.A. Tel: +39 02 96541 | Suomi/Finland Sandoz A/S Puh/Tel: +358 10 6133 400 |
Κ?προς Sandoz Pharmaceuticals d.d. Τηλ: +357 22 69 0690 | United Kingdom (Northern Ireland) Sandoz GmbH (Austria) Tel: +43 5338 2000 |
Latvija Sandoz d.d. Latvia filiale Tel: +371 67 892 006 |
- País de registro
- Disponibilidad en farmacias
Problema de suministro reportado
Los datos de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) indican un problema de suministro que afecta a este medicamento.<br><br>La disponibilidad puede ser limitada en algunas farmacias.<br><br>Para actualizaciones o alternativas, consulte a su farmacéutico. - Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a OMNITROPE 10 MG/1,5 ML SOLUCION INYECTABLE EN CARTUCHOForma farmacéutica: INYECTABLE, 12 mg somatropinaPrincipio activo: SomatropinaFabricante: Pfizer S.L.Requiere recetaForma farmacéutica: INYECTABLE, 5,3 mg somatropinaPrincipio activo: SomatropinaFabricante: Pfizer S.L.Requiere recetaForma farmacéutica: INYECTABLE, 0,2 mg somatropinaPrincipio activo: SomatropinaFabricante: Pfizer S.L.Requiere receta
Médicos online para OMNITROPE 10 MG/1,5 ML SOLUCION INYECTABLE EN CARTUCHO
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de OMNITROPE 10 MG/1,5 ML SOLUCION INYECTABLE EN CARTUCHO, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes