
NUCEIVA 50 UNIDADES POLVO PARA SOLUCIÓN INYECTABLE


Cómo usar NUCEIVA 50 UNIDADES POLVO PARA SOLUCIÓN INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
NUCEIVA 50 Unidades polvo para solución inyectable
toxina botulínica de tipo A
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es NUCEIVA y para qué se utiliza
- Qué necesita saber antes de empezar a usar NUCEIVA
- Cómo usar NUCEIVA
- Posibles efectos adversos
- Conservación de NUCEIVA
- Contenido del envase e información adicional
1. Qué es NUCEIVA y para qué se utiliza
NUCEIVA contiene el principio activo toxina botulínica de tipo A.
Impide la contracción de los músculos, lo que provoca una parálisis temporal. Actúa bloqueando los impulsos nerviosos hacia los músculos en los que se ha inyectado.
NUCEIVA se utiliza para mejorar temporalmente el aspecto de las arrugas verticales entre las cejas. Se utiliza en adultos menores de 65 años de edad en los que las arrugas faciales tienen un importante impacto psicológico.
2. Qué necesita saber antes de empezar a usar NUCEIVA
No use NUCEIVA:
- si es alérgico a la toxina botulínica de tipo A o a alguno de los demás componentes de este medicamento (incluidos en la sección 6);
- si padece miastenia grave o síndrome de Eaton Lambert (enfermedades crónicas que afectan a los músculos);
- si tiene una infección o inflamación en los lugares de inyección propuestos entre las cejas y sobre ellas (como se indica en la figura 1).
Advertencias y precauciones
Muy rara vez se producen efectos adversos posiblemente relacionados con la diseminación de la toxina botulínica desde el lugar de inyección (p. ej., debilidad muscular, dificultad para tragar o entrada de alimentos o líquidos en las vías respiratorias). Los pacientes que reciben las dosis recomendadas pueden presentar debilidad muscular exagerada.
La inyección se ha asociado a dolor localizado, inflamación/hinchazón, sensibilidad anómala (parestesia), disminución de la sensibilidad (hipoestesia), dolor a la palpación, erupción cutánea (eritema), infección localizada, hemorragia y/o hematomas. El dolor y/o la ansiedad relacionados con la aguja han dado lugar a respuestas vasovagales, como palidez, náuseas, sudoración, visión borrosa, ritmo cardíaco rápido, aturdimiento y/o un descenso temporal en la presión arterial que provoca mareos o desmayos.
Visite a su médico inmediatamente si le resulta difícil tragar, hablar o respirar después del tratamiento.
- Este medicamento no está recomendado en pacientes que hayan tenido problemas para tragar (disfagia) y respirar recientemente o en el pasado, ya que impediría la administración segura del medicamento en opinión del médico.
- La administración demasiado frecuente o excesiva puede provocar la formación de anticuerpos. La formación de anticuerpos puede impedir que la toxina botulínica de tipo A actúe incluso para otros usos.
- Muy raramente, se puede producir una reacción alérgica tras la inyección de toxina botulínica.
Entre los síntomas se incluyen reacciones cutáneas, como urticaria, picor y piel enrojecida o pálida, hinchazón de los ojos, los labios, la boca o la garganta, pulso débil y rápido, mareos y sibilancias o falta de aliento
- Se puede producir caída del párpado después del tratamiento.
Informe a su médico si:
- ha tenido problemas con inyecciones previas de toxina botulínica;
- no observa ninguna mejoría significativa de las arrugas un mes después del primer ciclo de tratamiento;
- padece determinadas enfermedades que afectan al sistema nervioso (como esclerosis lateral amiotrófica o neuropatía motora);
- tiene inflamación en el lugar de inyección propuesto;
- los músculos que se van a inyectar son débiles o están deteriorados;
- tiene un trastorno hemorrágico, ya que la inyección puede causar hematomas.
Niños y adolescentes
No se recomienda el uso de este medicamento en menores de 18 años de edad.
Otros medicamentos y NUCEIVA
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
No se recomienda el uso de toxina botulínica asociada a antibióticos aminoglucósidos, espectinomicina u otros medicamentos que afecten a los impulsos nerviosos hacia el músculo.
Informe a su médico si le han inyectado recientemente un medicamento que contenga toxina botulínica (el principio activo de NUCEIVA), ya que esto puede aumentar excesivamente el efecto de este medicamento.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
No se recomienda el uso de este medicamento durante el embarazo ni en mujeres capaces de tener hijos que no utilicen métodos anticonceptivos.
Este medicamento no está recomendado en mujeres en periodo de lactancia.
Conducción y uso de máquinas
Se han descrito debilidad muscular, mareos y alteraciones visuales con este medicamento que pueden hacer peligrosa la conducción o el uso de máquinas. No conduzca ni utilice máquinas hasta que estos efectos hayan desaparecido.
NUCEIVA contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente
«exento de sodio».
3. Cómo usar NUCEIVA
Las dosis unitarias de NUCEIVA no son intercambiables con las utilizadas para otros preparados de toxina botulínica.
Este medicamento sólo debe ser inyectado por médicos con la debida cualificación y experiencia en el tratamiento de las arrugas del entrecejo con el ceño fruncido.
La dosis habitual de NUCEIVA es de 20 Unidades. Se le inyectará el volumen recomendado de 0,1 mililitros (ml) (4 Unidades) de este medicamento en cada uno de los 5 lugares de inyección.
La mejoría de la profundidad de las arrugas entre las cejas se produce generalmente a los pocos días del tratamiento.
El intervalo entre los tratamientos lo decidirá su médico.
Cómo se inyecta NUCEIVA
Este medicamento se inyecta en los músculos (por vía intramuscular), directamente en la zona afectada entre las cejas y sobre ellas.
Una vez reconstituido, NUCEIVA solo se debe utilizar para tratar a un único paciente durante una sola sesión.
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
En general, los efectos adversos aparecen en los primeros días después de la inyección y son pasajeros. La mayoría de los efectos adversos son de intensidad leve o moderada.
Si tiene alguna dificultad para respirar, tragar o hablar después de recibir este medicamento, póngase en contacto con su médico inmediatamente.
Si presenta habones, hinchazón, incluida hinchazón de la cara o la garganta, sibilancias, sensación de debilidad y falta de aliento, contacte inmediatamente con su médico ya que pueden ser síntomas de una reacción alérgica.
La probabilidad de sufrir un efecto adverso se describe en las siguientes categorías:
Frecuentes (pueden afectar hasta 1 de cada 10) | Dolor de cabeza, desequilibrio muscular que produce cejas elevadas o asimétricas, caída de párpados, hematomas en el lugar de inyección |
Poco frecuentes (pueden afectar hasta 1 de cada 100 personas) | Trastornos sensitivos, molestias en la cabeza, ojo seco, hinchazón de los párpados, hinchazón de los ojos, contracciones musculares, lugar de inyección: enrojecimiento, dolor, hormigueo |
Comunicación de efectos adversos
Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de NUCEIVA
Conservar en nevera (entre 2ºC y 8ºC)
Mantener fuera de la vista y del alcance de los niños.
Vial sin abrir
No utilice este medicamento después de la fecha de caducidad que aparece en el vial y la caja después de CAD.
6. Contenido del envase e información adicional
Composición de NUCEIVA
- El principio activo es: 50 Unidades de toxina botulínica de tipo A.
- Los demás componentes son albúmina humana y cloruro sódico.
Aspecto del producto y contenido del envase
NUCEIVA se presenta en forma de polvo blanco para solución inyectable en un vial de vidrio transparente.
Cada envase contiene 1 vial.
Titular de la autorización de comercialización y Fabricantes
Evolus Pharma B.V.
Apollolaan 151
1077 AR Amsterdam
Países Bajos
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización.
Fecha de la última revisión de este prospecto: mayo 2024.
ESTA INFORMACIÓN ESTÁ DESTINADA ÚNICAMENTE A MÉDICOS O PROFESIONALES DEL SECTOR SANITARIO:
Las Unidades de toxina botulínica no son intercambiables de un producto a otro. Las dosis recomendadas en Unidades son diferentes de las de otros preparados de toxina botulínica.
La reconstitución se debe realizar de acuerdo con las buenas prácticas clínicas, especialmente con respecto a la técnica aséptica. NUCEIVA se reconstituye con cloruro de sodio 9 mg/ml (0,9 %) para solución inyectable. Se extraen en una jeringa 1,25 ml de cloruro de sodio 9 mg/ml (0,9 %) para solución inyectable para obtener una solución reconstituida inyectable en una concentración de 4 Unidades/0,1 ml.
Cantidad de disolvente añadida al vial de 50 Unidades (cloruro de sodio 9 mg/ml (0,9%) para solución inyectable) | Dosis resultante (Unidades por 0,1 ml) |
1,25 ml | 4,0 U |
La parte central del tapón de goma se debe limpiar con alcohol. Inyectar lentamente el diluyente en el vial con una aguja a través del tapón de goma y girar suavemente el vial evitando la formación de burbujas. El vial se debe desechar si al hacerse el vacío no se introduce el diluyente en el vial. Una vez reconstituida, la solución inyectable se debe inspeccionar visualmente antes de su uso para comprobar que es una solución transparente e incolora libre de partículas.
NUCEIVA reconstituido (50 Unidades/1,25 ml) se inyecta con una aguja estéril de calibre 30. Se administran cuatro unidades (4 U/0,1 ml) en cada uno de los 5 lugares de inyección (ver figura 1): 2 inyecciones en cada músculo corrugador (cara interna inferior y cara interna superior) y 1 inyección en el músculo prócer, lo que representa una dosis total de 20 Unidades.
Figura 1 Puntos de inyección
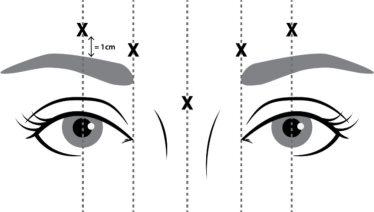
Para reducir la complicación de la ptosis palpebral, se deben adoptar las medidas siguientes:
- Evite la inyección cerca del elevador del párpado superior, sobre todo en los pacientes con complejos depresores de la ceja de gran tamaño.
- Las inyecciones en el corrugador lateral se deben aplicar al menos 1 cm por encima del borde supraorbitario óseo.
- Asegúrese de que el volumen/dosis inyectado es exacto y de que, cuando sea posible, se mantiene al mínimo.
Procedimiento para la eliminación segura de los viales, las jeringas y los materiales utilizados:
Inmediatamente después de su uso, la solución inyectable de NUCEIVA reconstituida no utilizada que quede en el vial o la jeringa se debe inactivar, antes de su eliminación, con 2 ml de solución de hipoclorito sódico diluido al 0,5 % o al 1 % de cloro disponible. Después de la inactivación, eliminar de acuerdo con los requisitos locales.
Los viales, las jeringas y los materiales usados no se deben vaciar y se deben desechar en recipientes apropiados y se deben eliminar de acuerdo con la normativa local.
Recomendaciones en caso de accidente durante la manipulación de la toxina botulínica:
En caso de accidente al manipular el producto, ya sea en estado de secado al vacío o reconstituido, se deberán aplicar inmediatamente las medidas apropiadas que se describen a continuación.
- La toxina es muy sensible al calor y a determinados agentes químicos.
- Los vertidos se deben limpiar con un material absorbente empapado en una solución de hipoclorito sódico (lejía) en el caso del producto secado al vacío o con un material absorbente seco si el producto ya está reconstituido.
- Las superficies contaminadas se deben limpiar con un material absorbente empapado en una solución de hipoclorito sódico (lejía) y secar a continuación.
- Si se rompe un vial, recoja cuidadosamente los trozos de vidrio y limpie el producto como se ha indicado anteriormente, evitando cortes en la piel.
- En caso de salpicaduras, lave con una solución de hipoclorito sódico y aclare a fondo con agua abundante.
- En caso de salpicarse en los ojos, lávelos bien con agua abundante o con una solución para lavado ocular.
- Si el operador se lesiona (se corta, se pincha), siga los pasos anteriores y tome las medidas médicas oportunas dependiendo de la dosis inyectada.
Se deben seguir estrictamente estas instrucciones de uso, manipulación y eliminación.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a NUCEIVA 50 UNIDADES POLVO PARA SOLUCIÓN INYECTABLEForma farmacéutica: INYECTABLE, 200 U/mlPrincipio activo: Botulinum toxinFabricante: Ipsen PharmaRequiere recetaForma farmacéutica: INYECTABLE, 125 Unidades SpeywoodPrincipio activo: Botulinum toxinFabricante: Ipsen Pharma S.A.U.Requiere recetaForma farmacéutica: INYECTABLE, 100 unidadesPrincipio activo: Botulinum toxinFabricante: Merz Pharmaceuticals GmbhRequiere receta
Médicos online para NUCEIVA 50 UNIDADES POLVO PARA SOLUCIÓN INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de NUCEIVA 50 UNIDADES POLVO PARA SOLUCIÓN INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















