
NITROFIX 10 mg PARCHES TRANSDERMICOS EFG


Cómo usar NITROFIX 10 mg PARCHES TRANSDERMICOS EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
NITROFIX 10 mg parches transdérmicos EFG
Nitroglicerina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es NITROFIX 10 mg y para qué se utiliza
- Qué necesita saber antes de empezar a tomar NITROFIX 10 mg
- Cómo usar NITROFIX 10 mg
- Posibles efectos adversos
- Conservación de NITROFIX 10 mg
- Contenido del envase e información adicional
1. Qué es NITROFIX 10 mg y para qué se utiliza
Los parches de NITROFIX 10 mg son sistemas transdérmicos consistentes en una película fina y transparente de polietileno de baja densidad, cubierta por una matriz adhesiva que contiene nitroglicerina. La matriz controla la velocidad de liberación del principio activo. El parche está recubierto por una película protectora de poliéster que se despega y desecha antes de su uso.
NITROFIX 10 mg está indicada para la prevención de la angina de pecho por esfuerzo y en reposo, asociado con insuficiencia coronaria.
2. Qué necesita saber antes de empezar a tomar NITROFIX 10 mg
No use NITROFIX10mg
- Si es alérgico (hipersensible) a los nitratos orgánicos o a cualquiera de los demás componentes de NITROFIX 10 mg.
- Si tiene anemia grave, hemorragia cerebral o traumatismos craneoencefálicos que cursen con hipertensión intracraneal o presión intraocular aumentada (glaucoma).
- En caso de Insuficiencia circulatoria aguda (shock, estados de colapso).
- Si está en tratamiento con medicamentos que contienen sildenafilo. Los pacientes en tratamiento con este medicamento nunca deben tomar conjuntamente medicamentos que contengan sildenafilo (medicamento empleado para alteraciones de la erección del pene). Para mayor información consulte con su médico o farmacéutico.
Tenga especial cuidado con NITROFIX10mg
NITROFIX10 mg debe utilizarse bajo estricta vigilancia médica en casos de infarto de miocardio o de insuficiencia cardiaca congestiva. Se recomienda extremar la precaución en el uso de NITROFIX 10 mg en pacientes con hipoxemia o desequilibrio de la perfusión de ventilación.
NITROFIX10 mg no está indicado en el tratamiento de ataques agudos de angina. En este caso su médico le prescribirá nitroderivados de acción rápida por vía sublingual.
Si se va a suspender el tratamiento, la dosis y frecuencia de la aplicación del parche NITROFIX 10 mg debe reducirse gradualmente durante un periodo de 4-6 semanas para evitar las reacciones de retirada de esta clase de fármacos vasodilatadores.
Este medicamento puede producir hipotensión postural, especialmente en pacientes ansiosos, por lo que evite cambios bruscos de postura.
Se recomienda retirar el parche antes de una cardioversión o desfibrilación.
Es posible la aparición de acostumbramiento a la preparación o a otros nitroderivados. Por este motivo, el médico puede recomendarle, para mantener los bajos niveles en plasma, aplicar NITROFIX 10 mg diariamente con un intervalo sin parche de 8 a 12 horas.
La nitroglicerina puede interferir en la medición de ciertos análisis clínicos (catecolaminas y ácido vanilicomandélico en la orina incrementando su excreción).
Informe inmediatamente a su médico si se produce cualquiera de las siguientes situaciones mientras usa NITROFIX 10 mg. Su médico decidirá si es necesario interrumpir el tratamiento con NITROFIX 10 mg.
Uso de otros medicamentos
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente otros medicamentos, incluso los adquiridos sin receta.
Es conveniente que el médico conozca si está bajo tratamiento con otros medicamentos, ya que pueden potenciar el efecto depresor de la presión sanguínea de NITROFIX 10 mg (antagonistas del calcio, betabloqueantes, diuréticos, antihipertensivos, antidepresivos tricíclicos, tranquilizantes mayores y dihidroergotamina).
La administración con otros vasodilatadores debe hacerse con prudencia, con el objeto de evitar la adición de efectos.
No puede excluirse la posibilidad de que la ingestión de ácido acetilsalicílico y de sustancias antiinflamatorias no esteroideas pueda disminuir la respuesta terapéutica a NITROFIX 10 mg .
La acción de este medicamento sobre el corazón puede verse alterada si se utiliza conjuntamente con preparados que contengan sildenafilo para la erección del pene (ver contraindicaciones).
El uso de productos de aplicación tópica, especialmente si es prolongada, puede ocasionar fenómenos de sensibilización. En este caso deberá suspenderse el tratamiento, adoptándose las medidas terapéuticas oportunas.
Uso de NITROFIX10mg con los alimentos y bebidas
Debe evitarse la ingestión excesiva de alcohol.
Embarazo y lactancia
Consulte a su médico o farmacéutico antes de utilizar cualquier medicamento.
NITROFIX 10 mg debe emplearse con precaución durante el embarazo y la lactancia. En estas circunstancias, el producto resulta adecuado únicamente en caso de necesidad y bajo control directo de un médico. En caso de embarazo, notifíqueselo inmediatamente a su médico si está usando regularmente este medicamento.
Uso en niños:La seguridad y eficacia en menores de 18 años no ha sido establecida. Por tanto su uso no está recomendado.
Uso en ancianos:Los pacientes geriátricos suelen ser más sensibles a los efectos hipotensores.
Conducción y uso de máquinas
Debido a que muchos de los efectos secundarios potenciales (mareo, hipotensión, etc...) pueden disminuir la capacidad de reacción, deberán tomarse las máximas precauciones con la conducción de automóviles o manejo de maquinaria al inicio del tratamiento.
3. Cómo usar NITROFIX 10 mg
Siga exactamente las instrucciones de administración de NITROFIX 10 mg indicadas por su médico. Consulte a su médico o farmacéutico si tiene dudas.
La respuesta a los nitroderivados varía según el individuo y se debe prescribir en cada caso la dosis mínima eficaz. Debido a que la cantidad de nitroglicerina que libera el parche de NITROFIX 10 mg es constante, la dosis administrada depende únicamente del área de contacto del parche.
La dosis normal es, iniciar el tratamiento con un parche de NITROFIX 5 mg al día. Si es necesario, y en función de la tolerancia demostrada, se puede incrementar el tratamiento a un parche de NITROFIX 10 mg o incluso de NITROFIX 15 mg al día.
La aplicación puede ser por un periodo de tiempo continuado de 24 horas, o intermitentemente, incorporando un intervalo sin parche de 8 a 12 horas (normalmente por la noche).
INSTRUCCIONES PARA LA CORRECTA ADMINISTRACIÓN
Cada parche de NITROFIX 10 mg está contenido en una pequeña bolsa protectora sellada. La zona adhesiva está recubierta por una película protectora, que debe extraerse justo antes de la aplicación sobre la piel.
Aplicar el parche NITROFIX 10 mg sobre la piel en una zona limpia, seca, sana (sin restos de crema) y poco provista de vello.
El procedimiento correcto de aplicación es el siguiente:
1.- Abrir la bolsa, rasgándola por la marca y extraer el parche de su interior.
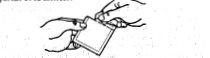
2.- Doblar ligeramente el parche con las marcas punteadas mirando hacia Vd, tirar de la lengüeta y desechar la película protectora.

3.- Aplicar la superficie con adhesivo en la parte superior del brazo o en el tórax. Separar con cuidado la otra parte punteada de la película protectora.

4.-Presionar sobre el parche, asegurar su buena colocación.
Retirar el parche NITROFIX 10 mg a las 24 horas, salvo otra recomendación del médico. Los parches NITROFIX 10 mg son desechables. Deben mantenerse fuera del alcance de los niños.
Aplicar un nuevo parche NITROFIX 10 mg siguiendo el método expuesto. Este nuevo parche debe aplicarse en una zona distinta a la del anterior, p.e. en el lado opuesto del pecho.
No aplicar el sistema en la misma zona durante días consecutivos. El parche NITROFIX 10 mg se adhiere con facilidad a la piel y no se desprende con el baño, la ducha o el ejercicio físico.
Si usa más NITROFIX10 mg del que debiera
Dosis elevadas de nitroglicerina pueden, en algunos casos, inducir un rápido descenso de la presión arterial y provocar shock, taquicardia, metahemoglobinemia, cianosis, coma y convulsiones. Debido a la liberación controlada de nitroglicerina con NITROFIX 10 mg , el riesgo de sobredosificación es muy raro. Cualquier reducción de la presión arterial y síntomas de colapso pueden tratarse elevando las extremidades inferiores. La metahemoglobinemia grave puede tratarse con una inyección de metiltionina o tolonio.
En caso de sobredosis o ingestión accidental, consultar al Servicio de Información Toxicológica. Teléfono: (91) 562 04 20.
Si olvidó usar NITROFIX10 mg
No tome una dosis doble para compensar las dosis olvidadas. Asegúrese de completar el ciclo de tratamiento.
Si interrumpe el tratamiento con NITROFIX10 mg
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, NITROFIX 10 mg puede producir efectos adversos, aunque no todas las personas los sufran.
La tolerancia a la nitroglicerina es, en general, buena. . El dolor de cabeza es el efecto secundario más frecuente, especialmente cuando se emplean dosis elevadas. Habitualmente este efecto se contrarresta con analgésicos comunes, aunque en casos particularmente intensos puede ser necesario reducir la dosis o interrumpir el tratamiento.
Otros posibles efectos secundarios que aparecen, sobre todo al inicio del tratamiento son: hipotensión arterial (especialmente postural), debilidad, taquicardia, desmayos, palpitaciones, sofocos, mareos, náuseas, vómitos y dermatitis. A excepción de la dermatitis, todos ellos son atribuibles a la acción farmacológica de la nitroglicerina. El médico debe ser inmediatamente informado de los efectos secundarios y de esta forma tener la oportunidad de suspender el tratamiento con NITROFIX 10 mg , al menos temporalmente.
La tolerancia local es también, en general, buena. Ocasionalmente se presenta, y siempre en función del nivel de aplicación, picor, escozor y ligeras reacciones de enrojecimiento. Sin embargo, estos efectos suelen desaparecer pocas horas después de retirar el parche sin necesidad de adoptar otras medidas.
Si considera que alguno de los efectos adversos que sufre es grave o si aprecia cualquier efecto adverso no mencionado en este prospecto, informe a su médico o farmacéutico.
5. Conservación de NITROFIX 10 mg
No requiere condiciones especiales de conservación
Mantener fuera del alcance y de la vista de los niños.
No utilice NITROFIX 10 mg después de la fecha de caducidad que aparece en el sobre o estuche, después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
No utilice NITROFIX 10 mg si observa indicios visibles de deterioro.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente..
6. Contenido del envase e información adicional
Composición de NITROFIX 10 mg
El principio activo es Nitroglicerina 53 mg liberando 10 mg/24 horas.
Los demás componentes son, DURO-tak 872852, sorbitan monooleato, propilenglicol, película oval de polietileno de baja densidad, película de poliester transparente.
Aspecto de NITROFIX 10 mg y contenido del envase
NITROFIX son parches transdérmicos transparentes de un área superficial de 13,3 cm2 y se presenta en envases de 7 ó 30 parches transdérmicos.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización:
ARAFARMA GROUP, S.A.
C/ Fray Gabriel de San Antonio, 6-10
Pol. Ind. del Henares
19180 Marchamalo (Guadalajara). España.
Responsable de la fabricación:
IBSA FARMACEUTICI ITALIA SRL
Strada Statale Nº 11, Padana Superiore, km 160
20051-Cassina de’ Pecchi (MI)
Italia
Ó
ALTERGON ITALIA SRL
Zona Industriale ASI,
Morra de Sanctis - 83040 (Av)
Italia
Este prospecto ha sido aprobado en abril de 2019.
- País de registro
- Precio medio en farmacia15.42 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a NITROFIX 10 mg PARCHES TRANSDERMICOS EFGForma farmacéutica: PARCHE TRANSDERMICO, 37,4 mg nitroglicerinaPrincipio activo: Trinitrato de gliceriloFabricante: Merus Labs Luxco Ii S.À.R.L.Requiere recetaForma farmacéutica: PARCHE TRANSDERMICO, 18,7 mg nitroglicerinaPrincipio activo: Trinitrato de gliceriloFabricante: Merus Labs Luxco Ii S.À.R.L.Requiere recetaForma farmacéutica: PARCHE TRANSDERMICO, 31,37 mgPrincipio activo: Trinitrato de gliceriloFabricante: Casen Recordati S.L.Requiere receta
Médicos online para NITROFIX 10 mg PARCHES TRANSDERMICOS EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de NITROFIX 10 mg PARCHES TRANSDERMICOS EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











