AREXVY powder and suspension for injectable suspension

How to use AREXVY powder and suspension for injectable suspension
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
Arexvy powder and suspension for suspension for injection
vaccine against respiratory syncytial virus (recombinant, adjuvanted)
This medicine is subject to additional monitoring, which will allow for quick identification of new safety information. You can help by reporting any side effects you may get. The last section of this leaflet includes information on how to report side effects.
Read all of this leaflet carefully before you start receiving this vaccine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others.
- If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the package leaflet
- What is Arexvy and what is it used for
- What you need to know before you receive Arexvy
- How Arexvy is administered
- Possible side effects
- Storage of Arexvy
- Contents of the pack and other information
1. What is Arexvy and what is it used for
Arexvy is a vaccine that helps protect adults aged 60 years and older against a virus called respiratory syncytial virus (RSV).
Arexvy also helps protect against RSV in adults aged 50 to 59 years with a higher risk of getting the disease.
RSV is a respiratory virus that spreads very easily.
- RSV can cause lower respiratory tract disease - infections in the lungs and other parts of the body that help you breathe.
RSV infection usually causes mild symptoms similar to those of a cold in healthy adults. However, it can also:
- cause more serious respiratory diseases and complications, such as lung infections (pneumonia), in older adults and adults with underlying diseases
- worsen some diseases, such as long-term respiratory or heart diseases.
How Arexvy works
Arexvy helps your body's natural defenses to produce antibodies and special white blood cells. These protect you against RSV.
Arexvy does not contain the virus. This means it cannot cause an infection.
2. What you need to know before you receive Arexvy
Do not use Arexvy
- if you are allergic to the active substances or to any of the other components of this vaccine (listed in section 6).
Do not use Arexvy if any of the above applies to you. If you are not sure, talk to your doctor or pharmacist.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before receiving Arexvy if:
- you have ever had a severe allergic reaction after being injected with any other vaccine
- you have a severe infection with a high temperature (fever). If this happens, vaccination may be delayed until you feel better. A minor infection, such as a cold, should not be a problem, but check with your doctor first
- you have a bleeding problem or bruise easily
- you have fainted after a previous injection. Fainting can occur before or after any injection with a needle.
If any of the above applies to you, or if you are not sure, talk to your doctor or pharmacist before Arexvy is administered.
As with all vaccines, Arexvy may not fully protect all people who are vaccinated.
Other medicines/vaccines and Arexvy
Tell your doctor or pharmacist if:
- you are taking, have recently taken, or might take any other medicines. This includes medicines obtained without a prescription
- you have recently received any other vaccine.
Arexvy can be given at the same time as a flu vaccine.
If Arexvy is given at the same time as another injectable vaccine, a different injection site will be used for each vaccine, i.e. a different arm for each injection.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before receiving this vaccine.
Arexvy is not recommended during pregnancy or breastfeeding.
Driving and using machines
Some of the effects mentioned below in section 4 "Possible side effects" (e.g. feeling tired) may temporarily affect your ability to drive or use machines. Do not drive or use machines or tools if you do not feel well.
Arexvy contains sodium and potassium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; this is essentially "sodium-free".
This medicine contains potassium, less than 1 mmol (39 mg) per dose; this is essentially "potassium-free".
3. How Arexvy is administered
Arexvy is given as a single injection of 0.5 ml into a muscle. It is usually given in the upper arm.
If you have any further questions on the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this vaccine can cause side effects, although not everybody gets them.
Serious side effects
Very rare(may affect up to 1 in 10,000 doses of vaccine):
- a neurological disorder that usually starts with a tingling sensation and weakness in the limbs and can progress to paralysis of one part or all of the body (Guillain-Barré syndrome).
Tell your doctor immediately if you notice signs of this serious side effect.
The following side effects may occur after receiving Arexvy:
Very common(may affect more than 1 in 10 doses of vaccine):
- pain at the injection site
- fatigue
- headache
- muscle pain
- joint pain
- redness at the injection site
Common(may affect up to 1 in 10 doses of vaccine):
- swelling at the injection site
- fever
- chills
Uncommon(may affect up to 1 in 100 doses of vaccine):
- itching at the injection site
- pain
- general feeling of being unwell
- enlargement of the lymph nodes, or swelling of the lymph nodes in the neck, armpits, or groin (lymphadenopathy)
- allergic reactions, such as rash
- nausea
- vomiting
- stomach pain
Not known(cannot be estimated from the available data):
- death of skin tissue at the injection site (necrosis at the injection site)
Tell your doctor or pharmacist if you get any of the side effects mentioned above. The intensity of most of these side effects is mild to moderate and does not last long.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet. You can also report side effects directly via the national reporting system listed in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Arexvy
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the label and carton, after EXP. The expiry date is the last day of the month stated.
- Store in a refrigerator (between 2°C and 8°C).
- Do not freeze.
- Store in the original package to protect from light.
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
Composition of Arexvy
- The active substances are:
After reconstitution, one dose (0.5 ml) contains:
RSVPreF3 antigen | 120 micrograms |
1 recombinant RSV F glycoprotein stabilised in a prefusion conformation = RSVPreF3
2 RSVPreF3 produced in Chinese Hamster Ovary (CHO) cells using recombinant DNA technology
3 adjuvanted with AS01E which contains: | |
Quillaja saponaria Molina fraction 21 (QS-21) plant extract | 25 micrograms |
3-O-desacetyl-4’-monophosphoryl lipid A (MPL) from Salmonella minnesota | 25 micrograms |
RSVPreF3 is a protein from the respiratory syncytial virus. This protein is not infectious.
The adjuvant is used to enhance the body's response to the vaccine.
- The other ingredients are:
- Powder(RSVPreF3 antigen): trehalose dihydrate, polysorbate 80 (E 433), potassium dihydrogen phosphate (E 340), and dipotassium phosphate (E 340).
- Suspension: dioleoyl phosphatidylcholine (E 322), cholesterol, sodium chloride, disodium phosphate anhydrous (E 339), potassium dihydrogen phosphate (E 340), and water for injections.
See section 2 "Arexvy contains sodium and potassium".
Appearance and pack contents
- Powder and suspension for injection.
- The powder is white.
- The suspension is an opalescent liquid, colorless to light brown.
One pack of Arexvy consists of:
- Powder (antigen) for 1 dose in a vial
- Suspension (adjuvant) for 1 dose in a vial
Arexvy is available in packs of 1 vial with powder and 1 vial with suspension or in packs of 10 vials with powder and 10 vials with suspension.
Not all pack sizes may be marketed.
Marketing authorisation holder and manufacturer
GlaxoSmithKline Biologicals SA
Rue de l’Institut 89
1330 Rixensart
Belgium
For further information on this medicine, contact the local representative of the marketing authorisation holder:
België/Belgique/Belgien GlaxoSmithKline Pharmaceuticals s.a./n.v Tel: + 32 (0) 10 85 52 00 | Lietuva GlaxoSmithKline Biologicals SA Tel: +370 80000334 |
| Luxembourg/Luxemburg GlaxoSmithKline Pharmaceuticals s.a./n.v Belgique/Belgien Tel: + 32 (0) 10 85 52 00 |
Ceská republika GlaxoSmithKline s.r.o. Tel: + 420 222 001 111 | Magyarország GlaxoSmithKline Biologicals SA Tel.: +36 80088309 |
Danmark GlaxoSmithKline Pharma A/S Tlf: + 45 36 35 91 00 | Malta GlaxoSmithKline Biologicals SA Tel: +356 80065004 |
Deutschland GlaxoSmithKline GmbH & Co. KG Tel: + 49 (0)89 360448701 | Nederland GlaxoSmithKline BV Tel: + 31 (0)33 2081100 |
Eesti GlaxoSmithKline Biologicals SA Tel: +372 8002640 | Norge GlaxoSmithKline AS Tlf: + 47 22 70 20 00 |
Ελλάδα GlaxoSmithKline Mοβπρóσωπη A.E.B.E. Τηλ: + 30 210 68 82 100 | Österreich GlaxoSmithKline Pharma GmbH Tel: + 43 (0)1 97075 0 |
España GlaxoSmithKline, S.A. Tel: + 34 900 202 700 | Polska GSK Services Sp. z o.o. Tel.: + 48 (22) 576 9000 |
France Laboratoire GlaxoSmithKline Tél : + 33 (0) 1 39 17 84 44 | Portugal GlaxoSmithKline – Produtos Farmacêuticos, Lda. Tel : + 351 21 412 95 00 |
Hrvatska GlaxoSmithKline Biologicals SA Tel.: +385 800787089 | România GlaxoSmithKline Biologicals SA Tel: +40 800672524 |
Ireland GlaxoSmithKline (Ireland) Ltd Tel: + 353 (0)1 495 5000 | Slovenija GlaxoSmithKline Biologicals SA Tel: +386 80688869 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika GlaxoSmithKline Biologicals SA Tel.: +421 800500589 |
Italia GlaxoSmithKline S.p.A. Tel: + 39 (0)45 7741 111 | Suomi/Finland GlaxoSmithKline Oy Puh/Tel: + 358 10 30 30 30 |
Κύπρος GlaxoSmithKline Biologicals SA Τηλ: +357 80070017 | Sverige GlaxoSmithKline AB Tel: + 46 (0)8 638 93 00 |
Latvija GlaxoSmithKline Biologicals SA Tel: +371 80205045 | United Kingdom (Northern Ireland) GlaxoSmithKline Biologicals SA Tel: +44(0)800 221441 |
Date of last revision of this leaflet:
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency web site: http://www.ema.europa.eu, and on the web site of the Spanish Agency for Medicines and Health Products (AEMPS) (http://www.aemps.gob.es/).
The leaflet can be found in all EU/EEA languages on the European Medicines Agency website.
<---------------------------------------------------------------------------------------------------------->
This information is intended only for healthcare professionals:
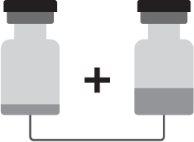
Arexvy is presented as a vial with a removable green mustard cap containing the powder (antigen) and a vial with a removable brown cap containing the suspension (adjuvant).
The powder and suspension must be reconstituted before administration.
Antigen | Adjuvant |
Powder | Suspension |
| |
1 dose (0.5 ml) |
Visually inspect the powder and suspension for any foreign particles and/or variation in appearance. If you notice any of these, do not reconstitute the vaccine.
How to prepare Arexvy
Arexvy must be reconstituted before administration.
- Withdraw the entire contents of the vial containing the suspension with a syringe.
- Add the entire contents of the syringe to the vial containing the powder.
- Gently shake until the powder is completely dissolved.
The reconstituted vaccine is an opalescent liquid, colorless to light brown.
Visually inspect the reconstituted vaccine for any foreign particles and/or variation in appearance. If you notice any of these, do not administer the vaccine.
Physical and chemical stability after reconstitution has been demonstrated for 4 hours between 2°C and 8°C or at room temperature up to 25°C.
From a microbiological point of view, the vaccine should be used immediately. If not used immediately, the in-use storage times and conditions are the responsibility of the user and should not exceed 4 hours.
Before administration
- Withdraw 0.5 ml of the reconstituted vaccine with the syringe.
- Change the needle so that a new needle is used.
Administer the vaccine by intramuscular injection.
Disposal of unused medicinal products and their waste should be in accordance with local requirements.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AREXVY powder and suspension for injectable suspensionDosage form: INJECTABLE, 60 micrograms/dose + 60 micrograms/doseActive substance: respiratory syncytial virus vaccinesManufacturer: Pfizer Europe Ma EeigPrescription requiredDosage form: INJECTABLE, 50 µgActive substance: respiratory syncytial virus vaccinesManufacturer: Moderna Biotech Spain S.L.Prescription requiredDosage form: INJECTABLE, 0.5 mlActive substance: ebola vaccinesManufacturer: Janssen-Cilag International N.VPrescription required
Online doctors for AREXVY powder and suspension for injectable suspension
Discuss questions about AREXVY powder and suspension for injectable suspension, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions