
MARAVIROC TARBIS 300 MG COMPRIMIDOS RECUBIERTOS CON PELICULA EFG

Cómo usar MARAVIROC TARBIS 300 MG COMPRIMIDOS RECUBIERTOS CON PELICULA EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Maraviroc Tarbis 150 mg comprimidos recubiertos con película EFG
Maraviroc Tarbis 300 mg comprimidos recubiertos con película EFG
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Maraviroc Tarbis y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Maraviroc Tarbis
- Cómo tomar Maraviroc Tarbis
- Posibles efectos adversos
- Conservación de Maraviroc Tarbis
- Contenido del envase e información adicional
1. Qué es Maraviroc Tarbis y para qué se utiliza y para qué se utiliza
Maraviroc contiene un principio activo llamado maraviroc. Maraviroc pertenece a un grupo de medicamentos llamados antagonistasdel CCR5. Maraviroc actúa bloqueando el receptor llamado CCR5, que el VIH utiliza para entrar e infectar las células sanguíneas.
Maraviroc se utiliza para tratar la infección por el Virus de la Inmunodeficiencia Humana tipo 1 (VIH-1) en adultos, adolescentes y niños de 2 años y mayores, que pesen al menos 10 kg.
Maraviroc debe tomarse en combinación con otros medicamentos que también son utilizados para tratar la infección por el VIH. A todos estos medicamentos se les denomina medicamentos anti-VIHo antirretrovirales.
Maraviroc, como parte del tratamiento combinado, reduce la cantidad de virus en su cuerpo y lo mantiene en un nivel bajo. Esto ayuda a su organismo a aumentar el recuento de células CD4 en la sangre. Las células CD4 son un tipo de glóbulo blanco que son importantes para ayudar a su cuerpo a combatir las infecciones.
2. Qué necesita saber antes de empezar a tomar Maraviroc Tarbis
No tome Maraviroc Tarbis
- si usted (o su hijo, si es el paciente) es alérgicoa maraviroc, al cacahuete, a la soja o a alguno de los demás componentes de maraviroc (incluidos en la sección 6).
?Consulte con su médicosi cree que esto le aplica a usted o a su hijo.
Advertencias y precauciones
Consulte con su médico o farmacéutico antes de tomar o administrar maraviroc.
Su médico debe recoger muestras de su sangre para ver si maraviroc es un tratamiento apropiado para usted (o su hijo, si es el paciente).
Algunas personas que toman maraviroc han desarrollado reacciones alérgicas graves o reacciones cutáneas (ver también “Efectos adversos graves” en la sección 4).
Antes de tomar este medicamento, asegúrese de que su médico conoce si usted (o su hijo) tiene o tuvo en el pasado alguno de los siguientes problemas:
- problemas de hígado, incluyendo hepatitiscrónica B o C. Sólo un número limitado de personas con problemas hepáticos han tomado maraviroc. Puede que su función hepática tenga que ser cuidadosamente vigilada. (Vea también “Problemas de hígado” en la sección 4).
- tensión arterial baja, incluyendo mareos cuando se pone de pie o se sienta rápidamente, o si está tomando medicamentos para bajar la tensión arterial. Esto es debido a una caída repentina en la tensión arterial. Si ocurre esto, túmbese (o a su hijo) hasta que se sienta mejor. Cuando se (le) incorpore, hágalo lo más lentamente posible.
- tuberculosis (TB)o una infección fúngicagrave. Maraviroc puede incrementar potencialmente su riesgo de desarrollar infecciones.
- problemas de riñón. Esto es particularmente importantesi también está tomando otros medicamentos (ver “Otros medicamentos y Maraviroc Tarbis” más adelante en la sección 2)
- problemas de corazóno del sistema circulatorio. Sólo un número limitado de personas con graves problemas de corazón o circulatorios han tomado maraviroc.
?Informe a su médicoantes de empezar el tratamiento si piensa que alguna de las situaciones anteriores le afecta a usted (o a su hijo).
Trastornos a los que debe estar atento
Algunas personas que toman medicamentos para la infección por el VIH desarrollan otros trastornos, que pueden ser graves. Estos incluyen:
- Síntomas de infecciones e inflamación
- Dolor en las articulaciones, rigidez y problemas óseos
Usted necesita conocer a qué signos y síntomas importantes debe prestar atención mientras está tomando maraviroc.
? Lea la información sobre “Otros posibles efectos adversos del tratamiento antirretroviral combinado frente al VIH” en la sección 4 de este prospecto.
Pacientes de edad avanzada
Sólo un número limitado de personas de 65 años o mayores han tomado maraviroc. Si usted pertenece a este grupo de edad, consulte con su médico si puede tomar maraviroc.
Niños
El uso de maraviroc no ha sido probado en niños menores de 2 años o con menos de 10 kg de peso. Por lo tanto, no se recomienda maraviroc en niños menores de 2 años o que pesen menos de 10 kg.
Otros medicamentos y Maraviroc Tarbis
Informe a su médico o farmacéutico si usted (o su hijo) están tomando, han tomado recientemente o pudieran tener que tomar cualquier otro medicamento.
Informe a su médico o farmacéutico si usted (o su hijo) comienza a tomar un nuevo medicamento mientras está tomando maraviroc.
Es probable que los medicamentos que contengan la Hierba de San Juan(Hypericum perforatum) impidan que maraviroc actúe correctamente. No debe tomarlo mientras esté tomando maraviroc.
Algunos medicamentos pueden afectar a los niveles de maraviroc en el organismo, cuando se toman al mismo tiempo que maraviroc. Estos incluyen:
- otros medicamentos para el tratamiento de la infección por el VIHo el virus de la hepatitis C(por ejemplo: atazanavir, cobicistat, darunavir, efavirenz, etravirina, fosamprenavir, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir, boceprevir, telaprevir)
- antibióticos(claritromicina, telitromicina, rifampicina, rifabutina)
- medicamentos antimicóticos(ketoconazol, itraconazol, fluconazol)
- medicamentos anticonvulsivantes(carbamazepina, fenitoína, fenobarbital).
? Informe a su médicosi usted (o su hijo) están tomando alguno de estos medicamentos. Esto permitirá a su médico prescribir la dosis correcta de maraviroc.
Embarazo y lactancia
Si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada:
? Consulte a su médicosobre los riesgos y beneficios de tomar maraviroc.
No se recomiendaque las mujeres que conviven con el VIH den el pecho porque la infección por VIH puede transmitirse al bebé a través de la leche materna.
Se desconoce si los componentes de maraviroc también pueden pasar a la leche materna. Si está dando el pecho o piensa en dar el pecho, debe consultar con su médico lo antes posible.
Conducción y uso de máquinas
Maraviroc puede hacerle sentir mareado.
? Noconduzca, monte en bicicleta ni maneje máquinas o herramientassi no está seguro de que no le afecta.
Maraviroc Tarbis contiene lecitina de soja.
No tome este medicamento si es alérgico al cacahuete o a la soja.
Maraviroc Tarbis contiene sodio.
Maraviroc contiene menos de 1 mmol de sodio (23 mg) en cada comprimido; esto es, esencialmente “exento de sodio”.
3. Cómo tomar Maraviroc Tarbis
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte a su médico o farmacéutico.
Si usted (o su hijo) son incapaces de tragar los comprimidos, su médico considerará si es mejor que tomen la solución oral de maraviroc.
Qué cantidad debe tomar
Adultos
La dosis recomendada de maraviroc es 150 mg, 300 mg o 600 mg dos veces al díadependiendo de si está usando otros medicamentos al mismo tiempo. Tome siempre la dosis recomendada por su médico.
Personas con problemas renales
Si usted tiene problemas renales, su médico le puede modificar su dosis.
?Consulte con su médicosi esto le aplica.
Adolescentes y niños de 2 años de edad en adelante y un peso de al menos 10 kg
Su médico le indicará la dosis correcta de maraviroc de acuerdo al peso y a otros medicamentos que estén siendo tomados al mismo tiempo.
Maraviroc Tarbis puede administrarse con o sin alimentos.Maraviroc siempre se debe tomar por vía oral.
Maraviroc debe tomarse en combinación con otros medicamentos utilizados para tratar el VIH. Consulte el prospecto de estos otros medicamentos para saber cómo tomarlos.
Si toma o administra más Maraviroc Tarbis del que debe
Si accidentalmente toma o administra demasiado maraviroc
?Contacte con su médico o acuda al hospital más cercano inmediatamente.
En caso de sobredosis o ingestión accidental, consulte con su médico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó tomar o administrar Maraviroc Tarbis
Si usted (o su hijo) olvidó tomar o administrar una dosis de maraviroc, tómela o adminístrela tan pronto como sea posible y continúe con la siguiente dosis a la hora habitual.
Si es casi la hora de la próxima dosis, no tome o administre la dosis olvidada. Espere a tomar la siguiente dosis a la hora que le corresponda.
No tome o administre una dosis doble para compensar las dosis olvidadas.
Si usted (o su hijo) interrumpe el tratamiento con Maraviroc Tarbis
Continúe el tratamiento con maraviroc hasta que su médico le indique.
Es importante que cada día tome sus medicamentos cuando le corresponda, ya que así se asegura que la infección por el VIH no se extienda por su organismo. Por lo tanto, a menos que su médico le indique detener el tratamiento, es importante que usted (o su hijo) continúe el tratamiento con maraviroc, como se ha descrito anteriormente.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, maraviroc puede producir efectos adversos, aunque no todas las personas los sufran. Informe a su médico sobre cualquier cambio inusual que se produzca en su salud o en la de su hijo.
Efectos adversos graves - busque ayuda médica inmediatamente
Reacciones alérgicas o cutáneas graves
Algunas personas que tomaban maraviroc han desarrollado reacciones cutáneas y reacciones alérgicas graves y potencialmente mortales. Estas son raras, pueden afectar hasta 1 de cada 1.000 personas que tomen maraviroc.
Si tiene alguno de estos síntomas mientras está siendo tratado con maraviroc:
- hinchazón de la cara, labios o lengua
- dificultad para respirar
- erupción cutánea generalizada
- fiebre (temperatura elevada)
- ampollas y descamación de la piel, especialmente alrededor de la boca, nariz, ojos y genitales
? Contacte con su médico inmediatamentesi tiene estos síntomas. Deje de tomar maraviroc.
Problemas de hígado
Éstos son raros y pueden afectar hasta 1 de cada 1.000 personasque tomen maraviroc. Los signos incluyen:
- pérdida de apetito
- náuseas/vómitos
- color amarillento de la piel u ojos
- erupción cutánea o picor
- sentirse muy cansado
- dolor estomacal o dolor a la palpación
- orina oscura
- somnolencia y confusión
- fiebre (temperatura elevada).
? Contacte con un médico inmediatamentesi tiene estos síntomas. Deje de tomar Maraviroc.
Otros efectos adversos
Efectos adversos frecuentes
Estos pueden afectar 1 a 10 de cada 100 personas:
- diarrea, malestar general, dolor de estómago, ventosidades (flatulencia)
- pérdida de apetito
- dolor de cabeza, dificultad para dormir, depresión
- erupción cutánea (vea también “Reacciones alérgicas o cutáneas graves” descritas anteriormenteen la sección 4)
- sensación de debilidad o falta de energía, anemia (observados en los análisis de sangre)
- aumento de enzimas hepáticas (observados en los análisis de sangre), lo que puede ser un signo de problemas de hígado (vea también “Problemas de hígado” descritos anteriormente en esta sección 4).
Efectos adversos poco frecuentes
Estos pueden afectar hasta 1 de cada 100 personas:
- infección de pulmón
- infección por hongos de la garganta (esófago)
- convulsiones
- sensación de mareo, debilidad o aturdimiento al ponerse de pie
- fallo del riñón, presencia de proteínas en la orina
- un incremento de una sustancia conocida como CPK (visto en los análisis de sangre) que es una señal de que los músculos estén inflamados o dañados.
Efectos adversos raros
Estos pueden afectar hasta 1 de cada 1.000 personas:
- dolor en el pecho (causado por la reducción del riego sanguíneo al corazón)
- disminución del tamaño de los músculos
- algunos tipos de cáncer como el de garganta (esófago) y del conducto biliar
- reducción en el número de células sanguíneas (observado en los análisis de sangre).
Otros posibles efectos adversos del tratamiento antirretroviral combinado frente al VIH
Las personas que toman tratamiento antirretroviral combinado frente al VIH pueden desarrollar otros efectos adversos.
Síntomas de infección e inflamación
Las personas con infección avanzada por el VIH (SIDA) tienen un sistema inmunitario debilitado y es más probable que desarrollen infecciones graves (infecciones oportunistas). Después de comenzar el tratamiento, el sistema inmunológico se vuelve más fuerte, de forma que el organismo empieza a luchar contra estas infecciones.
Se pueden desarrollar síntomas de infección e inflamación, causada ya sea por:
- infecciones antiguas, ocultas que brotan nuevamente cuando el organismo vuelve a luchar contra ellas
- el sistema inmunitario ataca tejidos sanos del cuerpo (trastornos autoinmunitarios).
Los síntomas de los trastornos autoinmunitariospueden aparecer muchos meses después de comenzar a tomar medicamentos para tratar la infección por el VIH. Los síntomas pueden incluir:
- debilidad muscular
- debilidad que empieza en las manos y pies y que asciende hacia el tronco del cuerpo
- palpitaciones o temblor
- hiperactividad (inquietud excesiva y movimiento).
Si tiene cualquier síntoma de infección o si usted nota alguno de los síntomas anteriores:
?Informe a su médico inmediatamente.No tome otros medicamentos para la infección sin el asesoramiento de su médico.
Dolor en las articulaciones, rigidez y problemas óseos
Algunos pacientes en tratamiento antirretroviral combinado frente al VIH desarrollan una afección llamada osteonecrosis. En esta afección, partes del tejido óseo mueren debido a una reducción del aporte de sangre a los huesos.
No se conoce la frecuencia de esta condición. Es más probable que se desarrolle:
- si ha tomado tratamiento combinado durante un largo tiempo
- si también está tomando medicamentos antiinflamatorios denominados corticoides
- si bebe alcohol
- si tiene un sistema inmunitario muy debilitado
- si tiene sobrepeso.
Los signos a tener en cuenta incluyen:
- rigidez en las articulaciones
- dolores (especialmente en cadera, rodilla u hombro)
- dificultad de movimiento.
Si nota cualquiera de estos síntomas:
? Informe a su médico.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es.
Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Maraviroc Tarbis
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y blister/frasco, después de CAD. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere condiciones especiales de conservación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia.
Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Maraviroc Tarbis
El principio activo es maraviroc.
Cada comprimido recubierto con película contiene 150 mg de maraviroc.
Cada comprimido recubierto con película contiene 300 mg de maraviroc.
Los demás componentes son:
Núcleo del comprimido: Celulosa microcristalina (E460), Carboximetilalmidón sódico (Tipo A), sílice coloidal anhidra, estearato de magnesio (E470b), hidrógenofosfato de calcio anhidro (E341).
Revestimiento: Poli (alcohol vinílico) (E1203), talco (E553b), dióxido de titanio (E171), macrogol (MW3350) (E1521), lecitina de Soja (E322), carmín de índigo (E132).
Aspecto del producto y contenido del envase
Maraviroc Tarbis 150 mg comprimidos recubiertos con película EFG
Comprimidos recubiertos con película, de color azul, ovalados, biconvexos, de dimensiones aproximadas 8,7 mm x 15,7 mm y grabados con “HM” en una cara y “150” en la otra.
Maraviroc Tarbis 300 mg comprimidos recubiertos con película EFG
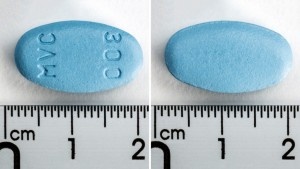
Comprimidos recubiertos con película, de color azul, ovalados, biconvexos, de dimensiones aproximadas 19,4 mm x 10,7 mm y grabados con “HM” en una cara y “300” en la otra.
Maraviroc Tarbis está disponible en blísteres que contienen 60 comprimidos recubiertos con película y en blísteres unidosis perforados que contienen 60 comprimidos recubiertos con película.
Frascos de HDPE que contiene 60 comprimidos recubiertos con película.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Tarbis Farma S.L.
Gran Vía Carlos III, 94
08028 Barcelona
España
Responsable de la fabricación
Amarox Pharma B.V.
Rouboslaan 32
Voorschoten, 2252TR
Países Bajos
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Alemania: Maraviroc Amarox 150 mg/300 mg Filmtabletten
Países Bajos: Maraviroc Amarox 150 mg/300 mg, filmomhulde tabletten
España: Maraviroc Tarbis 150 mg/300 mg comprimidos recubiertos con película EFG
Fecha de la última revisión de este prospecto: 02/2024
La información detallada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a MARAVIROC TARBIS 300 MG COMPRIMIDOS RECUBIERTOS CON PELICULA EFGForma farmacéutica: COMPRIMIDO, 150 mgPrincipio activo: MaravirocFabricante: Viiv Healthcare B.V.Requiere recetaForma farmacéutica: COMPRIMIDO, 300 mgPrincipio activo: MaravirocFabricante: Viiv Healthcare B.V.Requiere recetaForma farmacéutica: COMPRIMIDO, 150 mgPrincipio activo: MaravirocFabricante: Tarbis Farma S.L.Requiere receta
Médicos online para MARAVIROC TARBIS 300 MG COMPRIMIDOS RECUBIERTOS CON PELICULA EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de MARAVIROC TARBIS 300 MG COMPRIMIDOS RECUBIERTOS CON PELICULA EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















