
MABTHERA 1400 MG SOLUCION PARA INYECCION SUBCUTANEA

Cómo usar MABTHERA 1400 MG SOLUCION PARA INYECCION SUBCUTANEA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: Información para el paciente
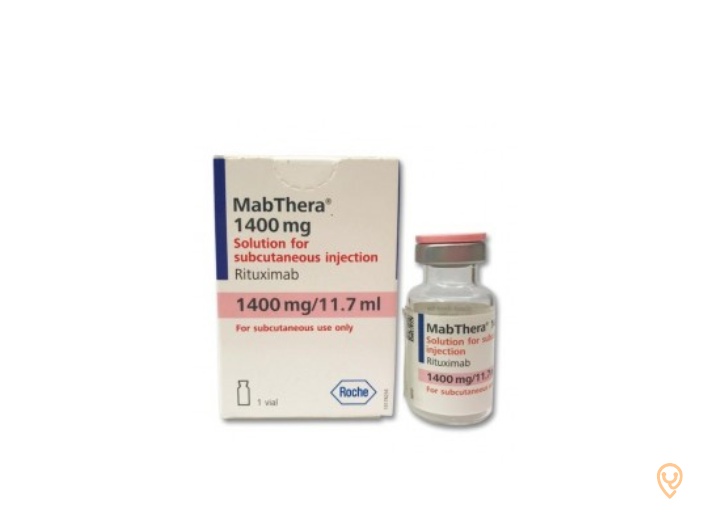
MabThera 1400mg solución para inyección subcutánea
rituximab
Lea todo el prospecto detenidamente antes de empezar a usar el medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es MabThera y para qué se utiliza
- Qué necesita saber antes de empezar a usar MabThera
- Cómo usar MabThera
- Posibles efectos adversos
- Conservación de MabThera
- Contenido del envase e información adicional
1. Qué es MabThera y para qué se utiliza
Qué es MabThera
MabThera contiene el principio activo “rituximab”. Esto es un tipo de proteína llamada “anticuerpo monoclonal”. Se une a la superficie de un tipo de glóbulos blancos llamados “linfocitos B”. Cuando rituximab se une a la superficie de estas células, provoca su muerte.
MabThera está disponible como medicamento para que se administre mediante goteo (llamado MabThera 100 mg o MabThera 500 mg concentrado para solución para perfusión), y como medicamento para que se administre como una inyección bajo su piel (llamado MabThera 1400 mg o MabThera 1600 mg solución para inyección subcutánea).
Para qué se utiliza MabThera
MabThera 1400 mg se usa para el tratamiento del Linfoma no-Hodgkin en adultos.
- Es una enfermedad del sistema linfático (parte del sistema inmunitario) que afecta a un tipo de glóbulos blancos, llamados linfocitos B.
MabThera 1400 mg puede administrarse sólo o con otros medicamentos llamados “quimioterapia”.
En el inicio de su tratamiento siempre se le administrará MabThera mediante goteo (perfusión intravenosa).
Después de esto, MabThera le será administrado como inyección bajo su piel. Su médico decidirá cuando empezar a utilizar MabThera como inyección.
En pacientes en los que haya funcionado el tratamiento, MabThera puede utilizarse como tratamiento de mantenimiento durante 2 años tras completar el tratamiento inicial.
2. Qué necesita saber antes de empezar a usar Mabthera
No use MabThera
- si es alérgico al rituximab, a otras proteínas similares a rituximab, o cualquiera de los demás componentes de este medicamento (incluidos en la sección 6)
- si es alérgico a la hialuronidasa (una enzima que ayuda a aumentar la absorción del principio activo)
- si tiene alguna infección activa, grave
- si tiene un sistema inmunitario débil.
No use MabThera si tiene alguno de los puntos anteriores. Si no está seguro, pregunte a su médico, farmacéutico o enfermero antes de que le administren MabThera.
Advertencias y precauciones
Informe a su médico, farmacéutico o enfermero antes de usar MabThera:
- si piensa que tiene una hepatitis infecciosa o la ha tenido en el pasado. Esto es porque en unos pocos casos, pacientes que habían tenido hepatitis B, pueden sufrir una recaída que ha sido fatal en muy raras ocasiones. Los pacientes con antecedentes de infección por hepatitis B serán vigilados rigurosamente por su médico para detectar posibles signos de hepatitis B
- si ha tenido alguna enfermedad cardiaca (tales como, angina de pecho, palpitaciones o fallo cardíaco) o problemas respiratorios.
Si le afecta alguno de los puntos anteriores (o no está seguro), pregunte a su médico, farmacéutico o enfermero antes de que le administren MabThera. Su médico deberá hacer seguimiento durante su tratamiento con MabThera.
Pregunte también a su médico si piensa que puede necesitar vacunarse en un futuro cercano, incluidas las vacunas necesarias para viajar a otros países. Algunas vacunas no deben ser administradas al mismo tiempo que MabThera o en los meses siguientes a su administración. Su médico comprobará si necesita alguna vacuna antes de recibir MabThera.
Niños y adolescentes
Informe a su médico, farmacéutico o enfermero antes de que le administren MabThera si usted o su niño tiene menos de 18 años. Esto es porque no hay mucha información sobre el uso de MabThera en niños y adolescentes.
Otros medicamentos y MabThera
Informe a su médico o farmacéutico si está utilizando o ha utilizado recientemente otros medicamentos, incluso los adquiridos sin receta. Esto es porque MabThera puede afectar al modo en que actúan otros medicamentos. También, otros medicamentos pueden afectar al modo en que MabThera actúa.
En particular informe a su médico si:
- si está en tratamiento para la hipertensión. Puede que le digan que no tome sus medicamentos durante las 12 horas anteriores a que le administren MabThera. Esto es porque algunas personas sufren una caída de tensión durante la perfusión.
- si ha tomado alguna vez medicamentos que afecten a su sistema inmunitario – tales como quimioterapia o medicamentos inmunosupresores.
Si le aplica alguno de los puntos anteriores (o no está seguro), pregunte a su médico, farmacéutico o enfermero antes de que le administren MabThera.
Embarazo y lactancia
Debe advertir a su médico si está embarazada, si cree que podría estar embarazada o si tiene intención de quedarse embarazada. Esto es debido a que MabThera puede cruzar la barrera placentaria y afectar a su bebé.
Si está en edad fértil, usted y su pareja deben utilizar un método anticonceptivo eficaz durante el tratamiento con MabThera y hasta 12 meses después del último tratamiento con MabThera.
MabThera pasa a la leche materna en muy pequeñas cantidades. Como se desconocen los efectos a largo plazo sobre los lactantes, por precaución no se recomienda la lactancia materna durante el tratamiento con Mabthera ni en los 6 meses posteriores al tratamiento.
Conducción y uso de máquinas
Se desconoce si MabThera tiene algún efecto sobre la capacidad para conducir vehículos o manejar máquinas.
MabThera contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg por vial de 11,7 ml, por lo que se considera esencialmente “exento de sodio”.
3. Cómo usar Mabthera
Cómo se usa este medicamento
MabThera le será administrado por un médico o enfermero con experiencia en el uso de este medicamento. Le mantendrá en observación durante la administración de MabThera por si sufre algún efecto adverso.
Al principio de su tratamiento siempre se le administrará MabThera mediante goteo (perfusión intravenosa).
Después, MabThera le será administrado como inyección bajo su piel (inyección subcutánea), durante aproximadamente 5 minutos. Se incluye un adhesivo en los viales que especifica la medicación.
Su médico o enfermera colocorá el adhesivo en la jeringa antes de la inyección.
Su médico decidirá cuando empezar a administrarle las inyecciones de MabThera.
La inyección de MabThera bajo su piel se hará en la zona abdominal, no en otras zonas de su cuerpo, y nunca en zonas abdominales donde la piel esté enrojecida, amoratada, blanda, dura o en zonas donde tenga lunares o cicatrices.
Medicamentos administrados antes de cada administración de MabThera
Antes de la administración de MabThera se le administrarán medicamentos (pre-medicación) para prevenir o reducir posibles efectos adversos.
Cantidad y frecuencia del tratamiento
- MabThera le será administrado el mismo día que la quimioterapia. Generalmente se administra hasta 8 veces en intervalos de 3 semanas.
- Si responde bien al tratamiento, podrá seguir en tratamiento con MabThera como mantenimiento cada 2 o 3 meses durante dos años.
Su médico podrá modificarlo dependiendo de su respuesta al medicamento.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, MabThera puede producir efectos adversos, aunque no todas las personas los sufran.
La mayor parte de estos efectos adversos son de intensidad leve a moderada, pero algunos de ellos pueden ser graves y requerir tratamiento. En casos raros algunas de estas reacciones han sido mortales.
Reacciones en el lugar de la inyección
Muchos pacientes tienen efectos adversos locales en el lugar de la inyección de MabThera. Estos incluyen: dolor, hinchazón, hematomas, sangrados, picor o erupción cutánea.
Su médico puede decidir no continuar su tratamiento con MabThera si tiene reacciones graves a la perfusión.
Infecciones
Advierta a su médico inmediatamente si tiene algún síntoma de infección, como:
- fiebre, tos, dolor de garganta, escozor al orinar, o si comienza a sentir cansancio o malestar general,
- pérdidas de memoria, problemas de concentración, dificultad para caminar o pérdida de visión. Esto puede deberse a una muy rara infección grave en el cerebro , la cual ha sido mortal (Leucoencefalopatía Multifocal Progresiva o LMP),
- fiebre, dolor de cabeza, rigidez de nuca, descoordinación (ataxia), cambio de personalidad, alucinaciones, alteración de la conciencia, convulsiones o coma – esto se podría deber a una infección grave del cerebro (meningoencefalitis enteroviral), que puede ser mortal.
Puede contraer infecciones más fácilmente durante el tratamiento con MabThera. Normalmente son resfriados, pero se han comunicado casos de neumonía o de infecciones urinarias. Todas ellas están enumeradas más abajo como “Otros efectos adversos”.
Otros efectos adversos
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas):
- infecciones bacterianas o víricas, bronquitis,
- número bajo de glóbulos blancos con o sin fiebre o de células sanguíneas llamadas “plaquetas”
- naúseas,
- calvas en el cuero cabelludo, escalofríos, dolor de cabeza,
- menor inmunidad por disminuir el número de anticuerpos llamados “inmunoglobulinas” (IgG) en la sangre que ayudan a proteger contra la infección.
Frecuentes (pueden afectar hasta 1 de cada 10 personas):
- infecciones de la sangre (sepsis), neumonía, herpes, resfriado, infecciones de los bronquios, infecciones por hongos, infecciones de origen desconocido, inflamación de los senos nasales, hepatitis B,
- bajo número de glóbulos rojos (anemia), bajo número de todas las células de la sangre,
- reacciones alérgicas (hipersensibilidad),
- altos niveles de azúcar en sangre, pérdida de peso, edemas periféricos y faciales, aumento de los niveles de enzima LDH en sangre, disminución de los niveles de calcio en la sangre,
- sensaciones anormales en la piel, así como entumecimiento, hormigueo, pinchazos, quemazón, aumento progresivo de estas sensaciones en la piel, disminución del sentido del tacto,
- agitación, dificultad para quedarse dormido,
- enrojecimiento de la cara y otras zonas de la piel como consecuencia de la dilatación de los vasos sanguíneos,
- sensación de mareo o ansiedad,
- aumento del lagrimeo, alteraciones en el conducto lacrimal, inflamación de los ojos (conjuntivitis),
- zumbido en el oído, dolor de oído,
- alteraciones cardiacas, como infarto de miocardio, irregular velocidad de latido, latidos anormalmente rápidos,
- aumento o disminución de la tensión (disminución en la tensión sobre todo al incorporarse),
- tensión de los músculos de las vías respiratorias que causa dificultad para respirar (broncoespasmo), inflamación, irritación en los pulmones, garganta y/o cavidades nasales, falta de aire, moqueo nasal,
- vómitos, diarrea, dolor abdominal, irritación o ulceraciones en la garganta y la boca, dificultades al tragar, estreñimiento, indigestión,
- alteraciones alimentarias: no comer suficiente, conduciendo a una pérdida de peso,
- habones, aumento de la sudoración, sudoración nocturna,
- problemas musculares, como tensión muscular, dolor en las articulaciones o músculos, dolor de espalda y cuello,
- dolor del tumor,
- malestar general o sensación de inquietud o cansancio, agitación, síntomas catarrales,
- insuficiencia multiorgánica.
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas):
- trastornos en la coagulación, disminución en la producción de glóbulos rojos, aumento de la destrucción de glóbulos rojos (anemia hemolítica aplásica), inflamación/ hinchazón de nódulos linfáticos,
- decaimiento, pérdida de interés por las actividades habituales, nerviosismo,
- alteraciones del sentido del gusto, tales como cambios en el sabor de los alimentos,
- problemas cardíacos, tales como reducción de la frecuencia cardiaca o dolor en el pecho (angina),
- asma, poca cantidad de oxígeno alcanza los órganos,
- hinchazón del estómago.
Muy raros (pueden afectar hasta 1 de cada 10.000 personas):
- aumento temporal de la cantidad de un tipo de anticuerpos en la sangre (llamados inmunoglobulinas – IgM), alteraciones químicas en la sangre causada por la ruptura de las células cancerosas,
- daño en nervios de brazos y piernas, parálisis de la cara,
- fallo del corazón,
- inflamación de los vasos sanguíneos, incluyendo las que conducen a los síntomas de la piel,
- insuficiencia respiratoria,
- daño en la pared del intestino (perforación),
- problemas graves en la piel que provocan ampollas que pueden amenazar la vida,
- problemas en el riñón (insuficiencia renal),
- pérdida de visión grave (signos de daño en nervios del cerebro).
Frecuencia no conocida(no se conoce la frecuencia con que ocurren estos efectos adversos):
- disminución retardada de glóbulos blancos en la sangre,
- reducción del número de plaquetas tras la perfusión- reversible, pero en casos raros puede ser mortal,
- pérdida de audición, pérdida de otros sentidos,
- infección/inflamación del cerebro y las meninges (meningoencefalitis enteroviral).
MabThera también puede causar cambios en las pruebas de laboratorio llevadas a cabo por su médico.
Si está en tratamiento con MabThera en combinación con otros medicamentos, algunos de los posibles efectos adversos pueden ser debidos a los otros medicamentos.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de MabThera
Mantener fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de EXP. La fecha de caducidad es el último día del mes que se indica.
Conservar en nevera (entre 2 ºC y 8 ºC). No congelar. Conservar el vial dentro del embalaje exterior para protegerlo de la luz.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de MabThera 1400mg solución para inyección subcutánea
- El principio activo de MabThera es rituximab. El vial contiene 1400 mg/11,7 ml de rituximab. Cada ml contiene 120 mg de rituximab.
- Los demás componentes son hialuronidasa recombinante humana (rHuPH20), L-histidina, L-histidina hidrocloruro monohidrato, ?,?-trehalosa dihidrato, L-metionina, polisorbato 80, agua para preparaciones inyectables. Ver sección 2 “MabThera contiene sodio”.
Aspecto del producto y contenido del envase
MabThera es una solución lista para usar, de transparente a opalescente, de incolora a amarillenta, que se presenta como una solución para inyección subcutánea en un vial de vidrio transparente con un tapón de goma de butilo sellado con aluminio y un disco flip-off de plástico rosa.
Cada vial contiene 1400 mg/11,7 ml de rituximab. Cada envase contiene un vial.
Titular de la autorización de comercialización
Roche Registration GmbH
Emil-Barell-Strasse 1
79639 Grenzach-Wyhlen
Alemania
Fabricante
Roche Pharma AG
Emil-Barrell-Str. 1
79639 Grenzach-Wyhlen
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización.

Ceská republika Roche s. r. o. Tel.: +420 - 2 20382111 | Magyarország Roche (Magyarország) Kft. Tel.: +36 - 1 279 4500 |
Danmark Roche Pharmaceuticals A/S Tel.: +45 - 36 39 99 99 | Malta (See Ireland) |
Deutschland Roche Pharma AG Tel.: +49 (0) 7624 140 | Nederland Roche Nederland B.V. Tel.: +31 (0) 348 438050 |
Eesti Roche Eesti OÜ Tel.: + 372 - 6 177 380 | Norge Roche Norge AS Tel.: +47 - 22 78 90 00 |
Ελλ?δα Roche (Hellas) A.E. Tel.: +30 210 61 66 100 | Österreich Roche Austria GmbH Tel.: +43 (0) 1 27739 |
España Roche Farma S.A. Tel: +34 - 91 324 81 00 | Polska Roche Polska Sp.z o.o. Tel.: +48 - 22 345 18 88 |
France Roche Tel: +33 (0) 1 47 61 40 00 | Portugal Roche Farmacêutica Química, Lda Tel.: +351 - 21 425 70 00 |
Hrvatska Roche d.o.o. Tel.: +385 1 4722 333 | România Roche România S.R.L. Tel.: +40 21 206 47 01 |
Ireland Roche Products (Ireland) Ltd. Tel.: +353 (0) 1 469 0700 | Slovenija Roche farmacevtska družba d.o.o. Tel.: +386 - 1 360 26 00 |
Ísland Roche Pharmaceuticals A/S c/o Icepharma hf Sími: +354 540 8000 | Slovenská republika Roche Slovensko, s.r.o. Tel.: +421 - 2 52638201 |
Italia Roche S.p.A. Tel.: +39 - 039 2471 | Suomi/Finland Roche Oy Puh/Tel: +358 (0) 10 554 500 |
K?προς Γ.Α.Σταμ?της & Σια Λτδ. Tel.: +357 - 22 76 62 76 | Sverige Roche AB Tel.: +46 (0) 8 726 1200 |
Latvija Roche Latvija SIA Tel.: +371 - 6 7039831 | United Kingdom (Northern Ireland) Roche Products (Ireland) Ltd. Tel: +44 (0) 1707 366000 |
Fecha de la última revisión de este prospecto
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
En la página web de la Agencia Europea de Medicamentos puede encontrarse este prospecto en todas las lenguas de la Unión Europea.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a MABTHERA 1400 MG SOLUCION PARA INYECCION SUBCUTANEAForma farmacéutica: INYECTABLE PERFUSION, 100 mgPrincipio activo: RituximabFabricante: Reddy Holding GmbhRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, 500 mgPrincipio activo: RituximabFabricante: Reddy Holding GmbhRequiere recetaForma farmacéutica: INYECTABLE, 100 mgPrincipio activo: RituximabFabricante: Roche Registration GmbhRequiere receta
Médicos online para MABTHERA 1400 MG SOLUCION PARA INYECCION SUBCUTANEA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de MABTHERA 1400 MG SOLUCION PARA INYECCION SUBCUTANEA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes






