LEQVIO 284 mg SOLUCION INYECTABLE EN JERINGA PRECARGADA

Cómo usar LEQVIO 284 mg SOLUCION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Leqvio 284mg solución inyectable en jeringa precargada
Jeringa precargada con protector de aguja
inclisirán
Lea todo el prospecto detenidamente antes de que le administren este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Leqvio y para qué se utiliza
- Qué necesita saber antes de que le administren Leqvio
- Cómo se administra Leqvio
- Posibles efectos adversos
- Conservación de Leqvio
- Contenido del envase e información adicional
1. Qué es Leqvio y para qué se utiliza
Qué es Leqvio y cómo funciona
Leqvio contiene el principio activo inclisirán. Inclisirán reduce los niveles de colesterol LDL (colesterol “malo”), que puede producir problemas de corazón y de circulación de la sangre cuando los niveles están elevados.
Inclisirán actúa interfiriendo con el ARN (encargado de trasladar la información genética de las células del cuerpo) para limitar la producción de una proteína llamada PCSK9. Esta proteína puede aumentar los niveles de colesterol LDL e impidiendo su producción se ayuda a reducir sus niveles de colesterol LDL.
Para qué se utiliza Leqvio
Leqvio se utiliza junto con su dieta de reducción del colesterol si usted es un adulto con nivel alto de colesterol en su sangre (hipercolesterolemia primaria, incluyendo la heterocigótica familiar y no familiar o dislipidemia mixta).
Leqvio se da:
- junto con una estatina (un tipo de medicamento que trata el colesterol alto), algunas veces combinado con otro tratamiento que baja el colesterol, si la dosis máxima de la estatina no funciona suficientemente bien, o
- solo o junto con otros medicamentos que bajan el colesterol cuando las estatinas no funcionan bien o no se pueden usar.
2. Qué necesita saber antes de que le administren Leqvio
No le deben administrar Leqvio
- si es alérgico a inclisirán o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de que le administren Leqvio:
- si está recibiendo diálisis
- si tiene una enfermedad hepática grave
- si tiene una enfermedad renal grave
Niños y adolescentes
No utilice este medicamento en niños y adolescentes de menos de 18 años de edad, porque no hay experiencia en el uso de este medicamento en este grupo de edad.
Otros medicamentos y Leqvio
Informe a su médico, farmacéutico o enfermero si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico, farmacéutico o enfermero antes de utilizar este medicamento.
Debe evitarse el uso de Leqvio durante el embarazo.
Todavía no se sabe si Leqvio puede pasar a la leche materna. Su médico le ayudará a decidir si continua la lactancia o si empieza el tratamiento con Leqvio. Su médico considerará los potenciales beneficios del tratamiento para usted, comparado con los beneficios para la salud y riesgos para la lactancia de su bebé.
Conducción y uso de máquinas
No se espera que Leqvio influya sobre su capacidad para conducir o utilizar máquinas.
Leqvio contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente “exento de sodio”.
3. Cómo se administra Leqvio
La dosis recomendada de Leqvio es de 284 mg administrado mediante una inyección bajo la piel (inyección subcutánea). La siguiente dosis se administra a los 3 meses y después las dosis adicionales cada 6 meses.
Antes de empezar con Leqvio usted debe de estar siguiendo una dieta para bajar su colesterol y probablemente esté tomando una estatina. Debería mantener la dieta para bajar el colesterol y seguir tomando la estatina durante todo el tiempo que reciba Leqvio.
Leqvio se administra como una inyección bajo la piel del abdomen, lugares alternativos de administración son la parte superior del brazo o el muslo. Leqvio se lo administrará un médico, farmacéutico o enfermero (profesional sanitario).
Si le han administrado más Leqvio del que se debe
Este medicamento se lo administrará un médico, farmacéutico o enfermero (profesional sanitario). En el caso improbable de que se le administre demasiado (una sobredosis) el médico u otro profesional sanitario seguirá sus efectos adversos.
Si olvidó que le administren Leqvio
Si olvidó una cita para que le administren su inyección de Leqvio, contacte con su médico, farmacéutico o enfermero tan pronto como pueda para poder organizar su próxima inyección.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
- Reacciones en el sitio de inyección, como dolor, rojez o erupción.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Leqvio
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y en la caja después de CAD/EXP. La fecha de caducidad es el último día del mes que se indica.
Este medicamento no requiere condiciones especiales de conservación. No congelar.
Su médico, farmacéutico o enfermero comprobará este medicamento y lo desechará si contiene partículas.
Los medicamentos no se deben tirar por los desagües ni a la basura. Su médico, farmacéutico o enfermero tirará los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Leqvio
- El principio activo es inclisirán. Cada jeringa precargada contiene inclisirán sodio equivalente a 284 mg de inclisirán en 1,5 ml de solución. Cada ml contiene inclisirán sodio equivalente a 189 mg de inclisirán.
- Los demás componentes son agua para preparaciones inyectables, hidróxido de sodio (E524) (ver sección 2 “Leqvio contiene sodio”) y ácido fosfórico concentrado (E338).
Aspecto del producto y contenido del envase
Leqvio 284 mg solución inyectable en jeringa precargada es una solución clara, entre incolora y ligeramente amarilla, prácticamente libre de partículas.
Cada envase contiene una jeringa precargada con protector de aguja de un único uso.
Titular de la autorización de comercialización
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Irlanda
Responsable de la fabricación
Sandoz GmbH
Biochemiestrasse 10
6336 Langkampfen
Austria
Novartis Pharmaceutical Manufacturing GmbH
Biochemiestrasse 10
6336 Langkampfen
Austria
Novartis Pharma GmbH
Roonstrasse 25
90429 Nuremberg
Alemania
Novartis Pharma GmbH
Sophie-Germain-Strasse 10
90443 Nürnberg
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 | Lietuva SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
???????? Novartis Bulgaria EOOD ???.: +359 2 489 98 28 | Luxembourg/Luxemburg Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 |
Ceská republika Novartis s.r.o. Tel: +420 225 775 111 | Magyarország Novartis Hungária Kft. Tel.: +36 1 457 65 00 |
Danmark Novartis Healthcare A/S Tlf.: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Deutschland Novartis Pharma GmbH Tel: +49 911 273 0 | Nederland Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Eesti SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norge Novartis Norge AS Tlf: +47 23 05 20 00 |
Ελλ?δα Novartis (Hellas) A.E.B.E. Τηλ: +30 210 281 17 12 | Österreich Novartis Pharma GmbH Tel: +43 1 86 6570 |
España Novartis Farmacéutica, S.A. Tel: +34 93 306 42 00 | Polska Novartis Poland Sp. z o.o. Tel.: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tél: +33 1 55 47 66 00 | Portugal Novartis Farma ‑ Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Hrvatska Novartis Hrvatska d.o.o. Tel: +385 1 6274 220 | România Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenija Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Ísland Vistor ehf. Sími: +354 535 7000 | Slovenská republika Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Italia Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Suomi/Finland Novartis Finland Oy Puh/Tel: +358 (0)10 6133 200 |
Κ?προς Novartis Pharma Services Inc. Τηλ: +357 22 690 690 | Sverige Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvija SIA Novartis Baltics Tel: +371 67 887 070 | |
België/Belgique/Belgien Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 | Lietuva SIA Novartis Baltics Lietuvos filialas Tel: +370 5 269 16 50 |
Novartis Bulgaria EOOD ?eπ.: +359 2 489 98 28 | Luxembourg/Luxemburg Novartis Pharma N.V. Tél/Tel: +32 2 246 16 11 |
Ceská republika Novartis s.r.o. Tel: +420 225 775 111 | Magyarország Novartis Hungária Kft. Tel.: +36 1 457 65 00 |
Danmark Novartis Healthcare A/S Tlf.: +45 39 16 84 00 | Malta Novartis Pharma Services Inc. Tel: +356 2122 2872 |
Deutschland Novartis Pharma GmbH Tel: +49 911 273 0 | Nederland Novartis Pharma B.V. Tel: +31 88 04 52 111 |
Eesti SIA Novartis Baltics Eesti filiaal Tel: +372 66 30 810 | Norge Novartis Norge AS Tlf: +47 23 05 20 00 |
Ελλáδα Novartis (Hellas) A.E.B.E. Τηλ: +30 210 281 17 12 | Österreich Novartis Pharma GmbH Tel: +43 1 86 6570 |
España Novartis Farmacéutica, S.A. Tel: +34 93 306 42 00 | Polska Novartis Poland Sp. z o.o. Tel.: +48 22 375 4888 |
France Novartis Pharma S.A.S. Tél: +33 1 55 47 66 00 | Portugal Novartis Farma ‑ Produtos Farmacêuticos, S.A. Tel: +351 21 000 8600 |
Hrvatska Novartis Hrvatska d.o.o. Tel: +385 1 6274 220 | România Novartis Pharma Services Romania SRL Tel: +40 21 31299 01 |
Ireland Novartis Ireland Limited Tel: +353 1 260 12 55 | Slovenija Novartis Pharma Services Inc. Tel: +386 1 300 75 50 |
Ísland Vistor ehf. Sími: +354 535 7000 | Slovenská republika Novartis Slovakia s.r.o. Tel: +421 2 5542 5439 |
Italia Novartis Farma S.p.A. Tel: +39 02 96 54 1 | Suomi/Finland Novartis Finland Oy Puh/Tel: +358 (0)10 6133 200 |
Κúπρος Novartis Pharma Services Inc. Τηλ: +357 22 690 690 | Sverige Novartis Sverige AB Tel: +46 8 732 32 00 |
Latvija SIA Novartis Baltics Tel: +371 67 887 070 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
Esta información está destinada únicamente a profesionales sanitarios:
Leqvio 284 mg solución inyectable en jeringa precargada
Jeringa precargada con protector de aguja
inclisirán
Los profesionales sanitarios deben referirse a la Ficha Técnica para la información completa de prescripción.
Indicación(ver sección4.1 de la Ficha Técnica)
Leqvio está indicado en adultos con hipercolesterolemia primaria (heterocigótica familiar y no familiar) o dislipidemia mixta, como adyuvante de la dieta:
- en combinación con una estatina o una estatina y otros tratamientos hipolipemiantes en pacientes que no consiguen alcanzar los objetivos de C-LDL con la dosis máxima de una estatina o,
- sola o en combinación con otros tratamientos hipolipemiantes en pacientes que son intolerantes a las estatinas, o para aquellos para los que las estatinas están contraindicadas.
Posología (ver sección4.2 de la Ficha Técnica).
La dosis recomendada es de 284 mg de inclisirán en una única inyección subcutánea administrada en una dosis inicial, otra a los 3 meses y posteriormente cada 6 meses.
Dosis olvidadas
Si se retrasa la administración de una dosis planificada durante un periodo inferior a 3 meses, se debe administrar inclisirán y mantener la dosificación de acuerdo al calendario inicial del paciente.
Si se retrasa la administración de una dosis planificada durante un periodo superior a 3 meses, se debe iniciar un nuevo calendario de dosificación – se debe administrar inclisirán en una dosis inicial, otra a los 3 meses y posteriormente cada 6 meses.
Transición del tratamiento de anticuerpos monoclonales inhibidores de proproteína convertasa subtilisina/kexina tipo 9 (PCSK9)
Inclisirán se puede administrar inmediatamente después de la última dosis de un anticuerpo monoclonal inhibidor de PCSK9. Para mantener la reducción del colesterol de lipoproteína de baja densidad (C-LDL) se recomienda administrar inclisirán 2 semanas después de la última dosis del anticuerpo monoclonal inhibidor de PCSK9.
Poblaciones especiales
Edad avanzada
No son necesarios ajustes de dosis en pacientes de edad avanzada (ver sección 5.2 de la Ficha Técnica).
Insuficiencia hepática
No son necesarios ajustes de dosis en pacientes con insuficiencia hepática leve (clase Child-Pugh A) o moderada (clase Child-Pugh B). No hay datos disponibles en pacientes con insuficiencia hepática grave (clase Child-Pugh C) (ver sección 5.2 de la Ficha Técnica). Inclisirán se debe utilizar con precaución en pacientes con insuficiencia hepática grave.
Insuficiencia renal
No son necesarios ajustes de dosis en pacientes con insuficiencia renal leve, moderada o grave o pacientes con enfermedad renal terminal (ver sección 5.2 de la Ficha Técnica). La experiencia con inclisirán es limitada en pacientes con insuficiencia renal grave. Inclisirán se debe usar con precaución en estos pacientes. Ver sección 4.4 de la Ficha Técnica para precauciones en caso de hemodiálisis.
Población pediátrica
No se ha establecido todavía la seguridad y eficacia de inclisirán en niños menores de 18 años. No se dispone de datos.
Forma de administración (ver sección4.2 de la Ficha Técnica)
Vía subcutánea.
Inclisirán es de administración subcutánea en el abdomen, algunos lugares alternativos de inyección son la parte superior del brazo o el muslo. Las inyecciones no deben hacerse en áreas con enfermedad cutánea activa o con heridas como quemaduras de sol, erupción cutánea, inflamación o infecciones cutáneas.
Cada dosis de 284 mg se administra mediante una jeringa precargada. Cada jeringa precargada es de un único uso.
Inclisirán está planteado para administrarse por un profesional sanitario.
Contraindicaciones (ver sección4.3 de la Ficha Técnica)
Hipersensibilidad al principio activo o a alguno de los excipientes.
Advertencias y precauciones especiales de empleo (ver sección4.4 de la Ficha Técnica)
Hemodiálisis
No se ha estudiado el efecto de la hemodiálisis en la farmacocinética de inclisirán. Teniendo en cuenta que inclisirán se elimina por vía renal, no se debe realizar una hemodiálisis hasta al menos 72 horas después de la administración de Leqvio.
Conservación(ver sección6.4 de la Ficha Técnica)
No requiere condiciones especiales de conservación. No congelar.
Instrucciones de Uso de Leqvio jeringa precargada con protector de aguja
Esta sección contiene información sobre cómo inyectar Leqvio.
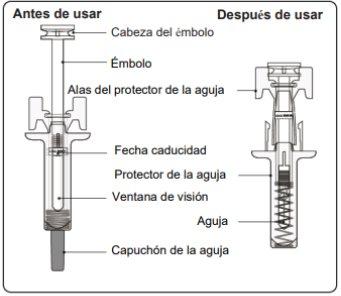
Información importante que necesita saber antes de inyectar Leqvio
- Noutilice la jeringa precargada si alguno de los sellos del envase exterior o el sello de la bandeja de plástico están rotos.
- Noquite el capuchón de la aguja hasta que esté preparado para inyectar.
- Noutilizar si la jeringa precargada se ha caído sobre una superficie dura o se ha dejado caer después de quitar el capuchón de la aguja.
- Nointente reutilizar o desmontar la jeringa precargada.
- La jeringa precargada tiene un protector de la aguja que se activará para cubrir la aguja cuando la inyección haya finalizado. El protector de la aguja ayudará a evitar heridas por pinchazo de aguja a cualquiera que manipule la jeringa precargada después de la inyección.
Paso 1. Inspeccionar la jeringa precargada
Podría ver burbujas de aire en el líquido, lo cual es normal. No intentequitar el aire.
- Noutilice la jeringa precargada si parece dañada o si parte de la solución inyectable se ha salido fuera de la jeringa precargada.
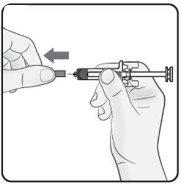
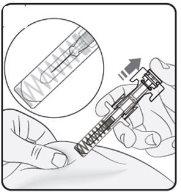
Paso 2. Quitar el capuchón de la aguja Estirar de manera firme y recta para quitar el capuchón de la aguja de la jeringa precargada. Podría ver una gota de líquido al final de la aguja. Es normal. Noponga otra vez el capuchón de la aguja. Tírelo. Noquite el capuchón de la aguja hasta que esté preparado para inyectar. La retirada temprana del capuchón antes de la inyección puede dar lugar al secado del producto terminado dentro de la aguja, lo que puede llevar a la obstrucción de esta. |
|
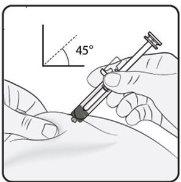
Paso 3. Inserte la aguja Pince suavemente la piel en el lugar de inyección y mantenga el pinzamiento durante la inyección. Con la otra mano inserte la aguja en la piel en un ángulo aproximado de 45 grados como se muestra en la imagen. |
|
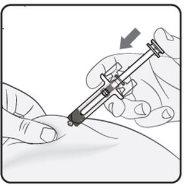
Paso 4. Inicio de la inyección Continúe pinzando la piel. Presione suavemente el émbolo tan lejos como se lo permita. Así se garantizará que se inyecta la dosis completa. Nota: Si no puede presionar el émbolo tras la inserción de la aguja, use una nueva jeringa precargada. |
|
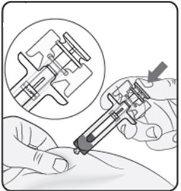
Paso 5. Complete la inyección Asegúrese de que la cabeza del émbolo está entre las alas del protector de la aguja como se muestra en la imagen. Así se garantizará que el protector de la aguja se ha activado y cubrirá la aguja una vez finalizada la inyección. |
|
Paso 6. Libere el émbolo Mientras mantiene la jeringa precargada en el lugar de inyección, libere despacio el émbolo hasta que la jeringa quede cubierta por el protector de la aguja. Quite la jeringa precargada del lugar de inyección. |
|
Paso 7. Deseche la jeringa precargada Deseche la jeringa precargada de acuerdo a la normativa local. |
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a LEQVIO 284 mg SOLUCION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: COMPRIMIDO, 10 mg ezetimibaPrincipio activo: ezetimibeFabricante: Organon Salud S.L.Requiere recetaForma farmacéutica: CAPSULA, 1.000 mgPrincipio activo: omega-3-triglycerides incl. other esters and acidsFabricante: Kern Pharma S.L.Requiere recetaForma farmacéutica: CAPSULA, 1000 mgPrincipio activo: omega-3-triglycerides incl. other esters and acidsFabricante: Strides Pharma (Cyprus) LimitedRequiere receta
Médicos online para LEQVIO 284 mg SOLUCION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de LEQVIO 284 mg SOLUCION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes