KIVIZIDIALE 40 MICROGRAMOS/ML + 5 MG/ML COLIRIO EN SOLUCION


Cómo usar KIVIZIDIALE 40 MICROGRAMOS/ML + 5 MG/ML COLIRIO EN SOLUCION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Kivizidiale 40 microgramos/ml + 5 mg/ml colirio en solución
Travoprost/timolol
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene informacion importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en el prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Kivizidiale y para qué se utiliza
- Qué necesita saber antes de empezar a usar Kivizidiale
- Cómo usar Kivizidiale
- Posibles efectos adversos
- Conservación de Kivizidiale
- Contenido del envase e información adicional
1. Qué es Kivizidiale y para qué se utiliza
Kivizidiale colirio en solución es una combinación de dos principios activos (travoprost y timolol). Travoprost es un análogo de prostaglandinas que actúa incrementando el flujo de salida del fluido acuoso del ojo, y por lo tanto disminuye la presión del ojo. Timolol es un betabloqueante que actúa reduciendo la formación de fluido en el interior del ojo. Las dos sustancias actúan de forma conjunta para reducir la presión en el interior del ojo.
Kivizidiale colirio se utiliza para el tratamiento de la presión elevada en el ojo en adultos, incluyendo pacientes de edad avanzada. Esta presión elevada puede causar una enfermedad denominada glaucoma.
Kivizidiale colirio en solución es una solución estéril que no contiene conservantes.
2. Qué necesita saber antes de empezar a usar Kivizidiale
No use Kivizidiale colirio en solución:
- Si es alérgico a travoprost, prostaglandinas, timolol, betabloqueantes o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- Si usted padece o ha padecido en el pasado problemas respiratorios como asma, bronquitis obstructiva crónica grave (enfermedad pulmonar grave que puede causar sibilancias, dificultad para respirar y/o tos persistente) u otros tipos de problemas respiratorios.
- Si padece rinitis alérgica grave.
- Si presenta ritmo cardíaco lento, insuficiencia cardíaca o un trastorno del ritmo cardíaco (latido cardíaco irregular).
- Si la superficie de su ojo está nublada.
Consulte a su médico si se encuentra en alguna de estas situaciones.
Advertencias y precauciones
Consulte a su médico antes de empezar a usar Kivizidiale si tiene o ha tenido en el pasado:
- Enfermedad coronaria (los síntomas pueden incluir dolor u opresión en el pecho, dificultad para respirar o sensación de ahogo), insuficiencia cardíaca, presión arterial baja.
- Alteraciones de la frecuencia cardíaca, como latido cardíaco lento.
- Problemas respiratorios, asma o enfermedad pulmonar obstructiva crónica.
- Trastornos por mala circulación sanguínea (como enfermedad de Raynaud o síndrome de Raynaud).
- Diabetes (ya que timolol puede enmascarar los signos y síntomas de niveles bajos de azúcar en sangre).
- Hiperactividad de la glándula tiroides (ya que timolol puede enmascarar los signos y síntomas de enfermedad de tiroides).
- Miastenia gravis (debilidad muscular crónica).
- Cirugía de cataratas.
- Inflamación ocular.
Si necesita someterse a algún tipo de operación, informe a su médico de que está utilizando Kivizidiale, ya que timolol puede modificar los efectos de algunos medicamentos usados durante la anestesia.
Si sufre cualquier reacción alérgica grave (erupción en la piel, enrojecimiento y picor en el ojo) mientras utiliza Kivizidiale, cualquiera que sea la causa, el tratamiento con adrenalina puede no ser tan eficaz. Por lo tanto, es importante que comunique al médico que está utilizando Kivizidiale cuando vaya a recibir cualquier otro tratamiento
Kivizidiale puede cambiar el color del iris (parte coloreada del ojo). Este cambio puede ser permanente.
Kivizidiale puede aumentar la longitud, el grosor, el color y/o el número de sus pestañas y puede causar un crecimiento inusual de pelo en sus párpados.
Travoprost puede absorberse por la piel y por tanto no debe utilizarse en mujeres embarazadas o que estén intentando quedarse embarazadas. Si este medicamento entra en contacto con la piel debe lavarse de inmediato.
Niños
Kivizidiale no debe utilizarse en niños y adolescentes menores de 18 años de edad.
Otros medicamentos y Kivizidiale
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento, incluyendo medicamentos que haya obtenido sin receta.
Kivizidiale puede afectar o verse afectado por otros medicamentos que esté usando, incluyendo otros colirios para el tratamiento del glaucoma. Informe a su médico si está usando o pretende usar:
- medicamentos para reducir la presión arterial,
- medicamentos para el corazón incluyendo quinidina (usada para tratar afecciones del corazón y algunos tipos de malaria),
- medicamentos para el tratamiento de la diabetes o antidepresivos conocidos como fluoxetina y paroxetina.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
No utilice Kivizidiale si está usted embarazada a menos que su médico así lo recomiende. Si usted puede quedarse embarazada debe utilizar un medio de contracepción adecuado mientras utilice este medicamento.
No utilice Kivizidiale si está en período de lactancia. Este medicamento puede pasar a la leche materna.
Conducción y uso de máquinas
Kivizidiale puede causar visión borrosa justo después de su uso. No conduzca ni use máquinas hasta que los síntomas hayan desaparecido.
Kivizidiale contiene hidroxiestearato de macrogolglicerol 40
Este medicamento contiene hidroxiestearato de macrogolglicerol 40, que puede producir reacciones cutáneas.
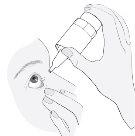
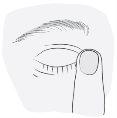
3. Cómo usar Kivizidiale
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada es
Una gota administrada una vez al día bien por la mañana o bien por la noche en el ojo o los ojos afectados. Utilice este medicamento todos los días a la misma hora.
Solo utilice Kivizidiale en los dos ojos si su médico así se lo ha indicado.
Utilice Kivizidiale tanto tiempo como le haya dicho su médico.
Kivizidiale solo debe utilizarse como colirio.
Si está utilizando otros colirios además de Kivizidiale, espere al menos 5 minutos entre la aplicación de Kivizidiale y las otras gotas.
Si utiliza lentes de contacto blandas, no utilice las gotas con las lentes puestas. Espere 15 minutos tras echarse las gotas antes de ponerse las lentes de contacto de nuevo.
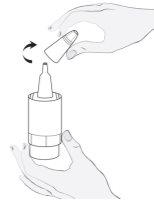
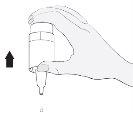
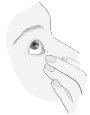
Instrucciones de uso
1a
1b |
|
2 |
|
3 |
|
4
5 |
|
|
Si usa más Kivizidiale del que debe
Si usa más Kivizidiale del que debe, enjuáguese los ojos con agua tibia. No aplique más gotas hasta que sea la hora de su dosis habitual.
En caso de sobredosis o ingestión accidental consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó usar Kivizidiale
Si olvidó aplicar Kivizidiale, continúe con la siguiente dosis como estaba planeado. No aplique una dosis doble para compensar la dosis olvidada. La dosis no debe exceder de 1 gota al día en el (los) ojo(s) afectado(s).
Si interrumpe el tratamiento con Kivizidiale
Si deja de utilizar Kivizidiale sin consultar con su médico, la presión en su ojo dejará de estar controlada, lo que podría provocar pérdida de visión.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
En general, a no ser que los efectos sean graves, usted puede continuar utilizando el colirio. Si tiene alguna duda, consulte con un médico o farmacéutico. No deje de usar Kivizidiale sin consultar a su médico.
Efectos adversos muy frecuentes(pueden afectar a más de 1 de cada 10 personas):
Efectos en el ojo
Enrojecimiento del ojo.
Efectos adversos frecuentes(pueden afectar a 1 de cada 10 personas):
Efectos en el ojo
Inflamación de la superficie ocular con daño en la superficie, dolor ocular, visión borrosa, visión anormal, ojo seco, picor en el ojo, molestias en el ojo, signos y síntomas de irritación ocular (p.ej.: quemazón, escozor).
Efectos adversos poco frecuentes(pueden afectar a 1 de cada 100 personas):
Efectos en el ojo
Inflamación de la superficie del ojo, inflamación del párpado, hinchazón de la conjuntiva, aumento del crecimiento de las pestañas, inflamación del iris, inflamación del ojo, sensibilidad a la luz, visión reducida, ojos cansados, alergia ocular, ojos hinchados, incremento de la producción de lágrimas, enrojecimiento del párpado, cambio del color del párpado, oscurecimiento de la piel (alrededor del ojo).
Otros efectos
Reacción alérgica a la sustancia activa, mareo, dolor de cabeza, aumento o disminución de la presión sanguínea, falta de aliento, crecimiento excesivo del vello, goteo en la parte posterior de la garganta, inflamación y picor de la piel, frecuencia cardíaca disminuida.
Efectos adversos raros(pueden afectar a 1 de cada 1.000 personas):
Efectos en el ojo
Adelgazamiento de la superficie ocular, inflamación de las glándulas del párpado, rotura de vasos sanguíneos en el ojo, costras en los párpados, disposición anormal de las pestañas, crecimiento anormal de las pestañas.
Otros efectos
Nerviosismo, frecuencia cardíaca irregular, pérdida de cabello, alteraciones de la voz, dificultad para respirar, tos, irritación de garganta, urticaria, valores anormales en las pruebas sanguíneas del hígado, decoloración de la piel, sed, cansancio, incomodidad dentro de la nariz, orina coloreada, dolor en manos y pies.
Frecuencia no conocida (no se puede estimar a partir de los datos disponibles):
Efectos en el ojo
Párpado caído (provocando que el ojo quede medio cerrado), ojos hundidos (los ojos parecen más profundos), cambios en el color del iris (parte coloreada del ojo).
Otros efectos
Erupción en la piel, insuficiencia cardíaca, dolor en el pecho, infarto, desvanecimiento, depresión, alucinación, asma, incremento del ritmo cardíaco, sensación de hormigueo o entumecimiento, palpitaciones, hinchazón en los miembros inferiores, mal sabor de boca.
Asimismo:
Kivizidiale es una asociación de 2 sustancias activas, travoprost y timolol. Al igual que otros medicamentos administrados en los ojos, travoprost y timolol (un betabloqueante) se absorben y pasan a la sangre. Esto puede causar efectos adversos similares a los observados con los medicamentos betabloqueantes administrados por la boca o por inyección. La incidencia de efectos adversos después de la administración en los ojos es inferior que cuando los medicamentos se administrán por la boca o inyectados.
Los efectos adversos enumerados a continuación incluyen reacciones observadas con la clase de betabloqueantes utilizados para tratar afecciones del ojo, o reacciones observadas con travoprost solo:
Efectos en el ojo
Inflamación del párpado, inflamación en la córnea, desprendimiento de la capa situada por debajo de la retina que contiene los vasos sanguíneos los cuales pueden causar alteraciones de la visión después de una cirugía de filtración, sensibilidad corneal disminuida, erosión corneal (daño en la cara anterior del globo ocular), visión doble, secreción del ojo, hinchazón alrededor del ojo, picor en el párpado, vuelta anormal hacia fuera del párpado con enrojecimiento, irritación y aumento en la producción de lágrimas, visión borrosa (signo de opacidad del cristalino), hinchazón de una parte del ojo (úvea), eczema de los párpados, visión de halos, sensibilidad disminuida en el ojo, pigmentación dentro del ojo, pupilas dilatadas, cambio en el color de las pestañas, cambio en la textura de las pestañas, campo visual anormal.
Otros efectos
- Trastornos del oído y del laberinto: mareos con sensación de vértigo, pitidos en los oídos.
- Corazón y circulación: frecuencia cardíaca lenta, palpitaciones, edema (acumulación de líquido), cambios en el ritmo o la frecuencia del latido cardíaco, insuficiencia cardíaca congestiva (enfermedad del corazón con dificultad para respirar e hinchazón de los pies y las piernas debido a la acumulación de líquidos), un tipo de trastorno del ritmo cardíaco, ataque al corazón, disminución de la tensión arterial, fenómeno de Raynaud, manos y pies fríos, reducción del riego sanguíneo al cerebro.
- Respiratorio: constricción de las vías respiratorias de los pulmones (principalmente en pacientes con enfermedad preexistente), nariz que moquea o taponada, estornudos (debido a la alergia), dificultad para respirar, sangrados de nariz, sequedad nasal.
- Trastornos generales y del sistema nervioso: dificultad para dormir (insomnio), pesadillas, pérdida de memoria, pérdida de fuerza y energía, ansiedad (angustia emocional excesiva).
- Trastornos gastrointestinales: alteración del gusto, náuseas, indigestión, diarrea, boca seca, dolor abdominal, vómitos, estreñimiento.
- Alergia: aumento de los síntomas alérgicos, reacciones alérgicas generalizadas incluyendo hinchazón bajo la piel que se puede producir en áreas tales como la cara y las extremidades, y pueden obstruir las vías aéreas y causar dificultad para tragar o respirar, erupción localizada y generalizada, picor, reacción alérgica repentina grave que puede comprometer la vida.
- Piel: erupción cutánea con aspecto blanco plateado (erupción psoriasiforme) o empeoramiento de la psoriasis, descamación de la piel, textura anormal del pelo, inflamación de la piel con sarpullido y enrojecimiento, cambio del color del pelo, pérdida de pestañas, picor, crecimiento anormal del pelo, enrojecimiento de la piel.
- Muscular: incremento de los signos y síntomas de la miastenia gravis (trastorno muscular), sensación inusual como de agujas o pinchazos, debilidad muscular/cansancio, dolor muscular no causado por el ejercicio, dolor en las articulaciones.
- Trastornos renales y urinarios: dificultad y dolor al orinar, pérdida de orina involuntaria.
- Reproducción: disfunción sexual, disminución de la líbido.
- Metabolismo: niveles bajos de azúcar en sangre, aumento del marcador del cáncer de próstata.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Kivizidiale
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta del frasco y en la caja después de «CAD». La fecha de caducidad es el último día del mes que se indica.
No utilice este medicamento si observa que el precinto de seguridad del frasco está roto antes de utilizarlo por primera vez.
No conservar a temperatura superior a 25ºC.
Para evitar infecciones, debe desechar el frasco del medicamento 28 días después de haberlo abierto por primera vezy utilizar un frasco nuevo. Escriba la fecha de apertura del frasco en la etiqueta del frasco y en la caja en el espacio designado para ello.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda, pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Kivizidiale
- Los principios activos son travoprost y timolol. Cada ml de solución contiene 40 microgramos de travoprost y 5 mg de timolol (como timolol).
- Los demás componentes son manitol (E421), ácido bórico, hidróxido de sodio (para el ajuste del pH), hidroxiestearato de macrogolglicerol (valor nominal: 40), propilenglicol (E1520), cloruro sódico y agua purificada.
Aspecto del producto Kivizidiale y contenido del envase
Kivizidiale colirio en solución se presenta como una solución acuosa de 2,5 ml, transparente, incolora y prácticamente libre de partículas, en un envase multidosis blanco (PP) de 5 ml con un sistema de bombeo (PP, HDPE, LDPE) y un cilindro de presión y tapón (HDPE) contenido en una caja de cartón.
El producto está disponible en los siguientes tamaños de envase:
Cajas que contienen 1 o 3 frascos.
Puede que no todos los tamaños de envase estén comercializados.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la Autorización de Comercialización:
BAUSCH + LOMB IRELAND LIMITED
3013 Lake Drive
Citywest Business Campus
Dublin 24, D24PPT3
Irlanda
Responsable de la Fabricación:
Pharmathen SA,
6 Dervenakion Str,
153 51 Pallini
Greece
O
JADRAN - GALENSKI LABORATORIJ d.d.
Svilno 20,
51000 Rijeka
Croatia
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del Titular de la Autorización de Comercialización.
Representante Local en España
Bausch & Lomb S.A.
Avda. Valdelaparra, 4
28108 – Alcobendas, Madrid
Tel: 91 – 657 63 00
Este medicamento está autorizado en los estados miembros del Espacio Econónimo Europeo con los siguientes nombres:
AT Kivizidiale 40 Mikrogramm/ml + 5 mg/ml Augentropfen Lösung
BE Kivizidiale 40 microgram/ml + 5 mg/ml oogdruppels, oplossing
BG ?????????? 40 ??????????/ml + 5 mg/ml ????? ?? ???, ???????
CY Kivizidiale
DE Kivizidiale 40 Mikrogramm/ml + 5 mg/ml Augentropfen, Lösung
DK Kivizidiale
EE Kivizidiale
ES Kivizidiale 40 μg/ml + 5 mg/ml colirio en solución
FR Kivizidiale 40 microgrammes/mL + 5 mg/mL, collyre en solution
EL Kivizidiale
HR Kivizidiale 40 mikrograma/ml + 5 mg/ml, kapi za oko, otopina
HU Kivizidiale 40 mikrogramm/ml + 5 mg/ml oldatos szemcsepp
NL Kivizidiale 40 microgram/ml + 5 mg/ml oogdruppels, oplossing
LT Kivizidiale 40 mikrogramu/ 5 mg/ ml akiu lašai (tirpalas)
LU Kivizidiale 40 microgrammes/ml + 5 mg/ml collyre en solution
PL Kivizidiale
PT Kivizidiale 40 μg/ml + 5 mg/ml colírio, solução
RO Kivizidiale 40 micrograme/mL + 5 mg/mL picaturi oftalmice, solu?ie
SK Kivizidiale 40 mikrogramov/ml + 5 mg/ml
Fecha de la última revisión de este prospecto: marzo 2022.
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/.
- País de registro
- Precio medio en farmacia7.84 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a KIVIZIDIALE 40 MICROGRAMOS/ML + 5 MG/ML COLIRIO EN SOLUCIONForma farmacéutica: COLIRIO, 10 mg/ml + 5 mg/mlPrincipio activo: timolol, combinationsFabricante: Novartis Europharm LimitedRequiere recetaForma farmacéutica: COLIRIO, 0,3 mg/ml + 5 mg/mlPrincipio activo: timolol, combinationsFabricante: Brill Pharma S.L.Requiere recetaForma farmacéutica: COLIRIO, 0,3 mg/ml+5mg/mlPrincipio activo: timolol, combinationsFabricante: Laboratorio Stada S.L.Requiere receta
Médicos online para KIVIZIDIALE 40 MICROGRAMOS/ML + 5 MG/ML COLIRIO EN SOLUCION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de KIVIZIDIALE 40 MICROGRAMOS/ML + 5 MG/ML COLIRIO EN SOLUCION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes