IMCIVREE 10 MG/ML SOLUCION INYECTABLE

Cómo usar IMCIVREE 10 MG/ML SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: Información para el paciente
IMCIVREE 10 mg/ml solución inyectable
setmelanotida
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es IMCIVREE y para qué se utiliza
- Qué necesita saber antes de empezar a usar IMCIVREE
- Cómo usar IMCIVREE
- Posibles efectos adversos
- Conservación de IMCIVREE
- Contenido del envase e información adicional
1. Qué es IMCIVREE y para qué se utiliza
IMCIVREE contiene el principio activo setmelanotida. Se utiliza en adultos y en niños a partir de
2 años para tratar la obesidad causada por determinadas enfermedades genéticas que afectan la forma en que el cerebro controla la sensación de hambre.
Las enfermedades genéticas en las que se utiliza este medicamento como tratamiento son:
- síndrome de Bardet-Biedl (SBB)
- obesidad por déficit de POMC (proopiomelanocortina)
- obesidad por déficit de PCSK1 (proproteína convertasa subtilisina/kexina tipo 1)
- obesidad por déficit de LEPR (receptores de leptina)
Las personas con estas enfermedades carecen de determinadas sustancias naturales que intervienen en el control del apetito o estas sustancias no funcionan correctamente. Esto aumenta los niveles de hambre y produce obesidad. Este medicamento ayuda a restablecer el control del apetito y reduce los síntomas de la enfermedad.
2. Qué necesita saber antes de empezar a usar IMCIVREE
No use IMCIVREE
- si es alérgico a la setmelanotida o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar IMCIVREE.
Antes de empezar el tratamiento con este medicamento, y mientras dure dicho tratamiento, su médico debe examinarle la piel para detectar marcas o zonas oscuras. Mientras usa este medicamento es posible que le aparezcan más marcas o manchas oscuras en la piel. Una revisión antes de iniciar el tratamiento le ayudará a identificar nuevas marcas que puedan aparecer una vez que haya usado este medicamento.
Es muy frecuente (puede afectar a más de 1 de cada 10 personas) que los pacientes varones experimenten erecciones espontáneas del pene al usar este medicamento. Si una erección dura más de 4 horas, consulte a un médico urgentemente. Las erecciones prolongadas (priapismo) pueden reducir su capacidad de tener erecciones en el futuro si no se tratan.
Niños
No administre este medicamento a niños menores de 2 años, ya que no se dispone de información sobre el uso en niños menores de esa edad.
Otros medicamentos e IMCIVREE
Informe a su médico o farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
Embarazo y lactancia
Si está embarazada o en período de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No se recomienda que las mujeres usen IMCIVREE si están embarazadas o intentan quedarse embarazadas, ya que no se ha estudiado en mujeres embarazadas. La pérdida de peso durante el embarazo puede perjudicar al bebé.
Consulte a su médico antes de empezar a tomar este medicamento si está dando el pecho. Su médico le explicará los beneficios y riesgos de tomar IMCIVREE durante este período.
Conducción y uso de máquinas
Este medicamento no debe tener efectos en su capacidad para conducir o usar máquinas.
IMCIVREE contiene alcohol bencílico
Este medicamento contiene 10 mg de alcohol bencílico en cada 1 ml, que equivalen a 1 mg por cada mg de su dosis.
El alcohol bencílico se ha asociado al riesgo de presentar efectos adversos graves en niños pequeños (de menos de 3 años). Existe una mayor probabilidad de que el alcohol bencílico se acumule en su cuerpo (lo que se llama «acidosis metabólica») y provoque «síndrome de jadeo». Los niños de 2 años deben ser supervisados por su médico para detectar esta acumulación (que se manifiesta con latidos cardíacos rápidos, respiración rápida o confusión).
El alcohol bencílico puede provocar reacciones alérgicas.
Consulte a su médico o farmacéutico si está embarazada o en periodo de lactancia. Esto es debido a que se pueden acumular grandes cantidades de alcohol bencílico en su organismo y provocar efectos adversos (acidosis metabólica).
Consulte a su médico o farmacéutico si tiene enfermedades de hígado o riñón. Esto es debido a que se pueden acumular en el organismo y provocar efectos adversos (acidosis metabólica).
IMCIVREE contiene sodio
Este medicamento contiene menos de 1 mmol de sodio (23 mg) por dosis; esto es, esencialmente
«exento de sodio».
3. Cómo usar IMCIVREE
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
IMCIVREE se administra en forma de inyección bajo la piel una vez al día al principio del día. Este medicamento está destinado a un uso de larga duración.
Su médico le aconsejará sobre la dosis adecuada que se debe inyectar.
Obesidad por déficit de proopiomelanocortina, obesidad por déficit de proproteína convertasa subtilisina/kexina tipo 1 y obesidad por déficit de receptores de leptina.
En adultos y niños a partir de los 12 años de edad, las dosis recomendadas son las siguientes:
Semana de tratamiento | Dosis diaria en mg | Volumen que se debe inyectar |
Semanas 1 – 2 | 1 mg una vez al día | 0,1 ml una vez al día |
Semana 3 en adelante | 2 mg una vez al día | 0,2 ml una vez al día |
Si la dosis no es suficiente y los efectos adversos son aceptables | 2,5 mg una vez al día | 0,25 ml una vez al día |
Si la dosis no es suficiente y los efectos adversos son aceptables | 3 mg una vez al día | 0,3 ml una vez al día |
En niños de 6 a < 12 años, las dosis recomendadas son las siguientes:
Semana de tratamiento | Dosis diaria en mg | Volumen que debe inyectarse |
Semanas 1 – 2 | 0,5 mg una vez al día | 0,05 ml una vez al día |
Semana 3 – 4 | 1 mg una vez al día | 0,1 ml una vez al día |
Semana 5 en adelante | 2 mg una vez al día | 0,2 ml una vez al día |
Si la dosis no es suficiente y los efectos adversos son aceptables | 2,5 mg una vez al día | 0,25 ml una vez al día |
En niños de 2 a <6años, las dosis recomendadas son las siguientes:
Peso del paciente/semana de tratamiento | Dosis diaria | Volumen que debe inyectarse |
<20kg | ||
Semana 1 en adelante | 0,5 mg una vez al día | 0,05 ml una vez al día |
20-<30kg | ||
Semanas 1-2 | 0,5 mg una vez al día | 0,05 ml una vez al día |
Semana 3 en adelante (si la dosis no es suficiente y los efectos adversos son aceptables) | 1 mg una vez al día | 0,1 ml una vez al día |
30-<40kg | ||
Semanas 1-2 | 0,5 mg una vez al día | 0,05 ml una vez al día |
Semanas 3-4 (si la dosis no es suficiente y los efectos adversos son aceptables) | 1 mg una vez al día | 0,1 ml una vez al día |
Semana 5 en adelante (si la dosis no es suficiente y los efectos adversos son aceptables) | 1,5 mg una vez al día | 0,15 ml una vez al día |
≥40kg | ||
Semanas 1-2 | 0,5 mg una vez al día | 0,05 ml una vez al día |
Semanas 3-4 (si la dosis no es suficiente y los efectos adversos son aceptables) | 1 mg una vez al día | 0,1 ml una vez al día |
Semanas 5-6 (si la dosis no es suficiente y los efectos adversos son aceptables) | 1,5 mg una vez al día | 0,15 ml una vez al día |
Semanas 7-8 (si la dosis no es suficiente y los efectos adversos son aceptables) | 2 mg una vez al día | 0,2 ml una vez al día |
Semana 9 en adelante (si la dosis no es suficiente y los efectos adversos son aceptables) | 2,5 mg una vez al día | 0,25 ml una vez al día |
Después de la dosis de inicio, si los efectos adversos de una dosis posterior no son aceptables, se reducirá la dosis al nivel de la dosis anterior. Si los efectos adversos de la dosis reducida son aceptables, se continuará el aumento de la dosis.
En pacientes con enfermedad renal leve o moderada, no se necesita ningún cambio en la pauta posológica.
Para adultos y niños de 12 a 17 años de edadcon insuficiencia renal grave, las dosis recomendadas son las siguientes:
Semana de tratamiento | Dosis diaria en mg | Volumen que debe inyectarse |
Semanas 1 – 2 | 0,5 mg una vez al día | 0,05 ml una vez al día |
Semana 3 en adelante (si los efectos adversos son aceptables) | 1 mg una vez al día | 0,1 ml una vez al día |
Si la dosis no es suficiente y los efectos adversos son aceptables | 2 mg una vez al día | 0,2 ml una vez al día |
Si la dosis no es suficiente y los efectos adversos son aceptables | 2,5 mg una vez al día | 0,25 ml una vez al día |
Si la dosis no es suficiente y los efectos adversos son aceptables | 3 mg una vez al día | 0,3 ml una vez al día |
Si los efectos adversos de la dosis inicial de 0,5 mg no son aceptables, se reducirá a 0,25 mg (0,025 ml). Si los efectos adversos de la dosis de 0,25 mg una vez al día son aceptables, continuará el aumento de la dosis.
Después de la dosis inicial, si los efectos adversos de una dosis posterior no son aceptables, se reducirá la dosis al nivel de dosis anterior. Si los efectos adversos de la dosis reducida son aceptables, continuará el aumento de la dosis.
Si los efectos adversos de la dosis de 3 mg no son aceptables, se reducirá a 2,5 mg y se le seguirá administrando esta dosis.
En niños de 6 a menos de 12 años de edadcon insuficiencia renal grave, las dosis recomendadas son las siguientes:
Semana de tratamiento | Dosis diaria en mg | Volumen que debe inyectarse |
Semanas 1 – 2 | 0,25 mg una vez al día | 0,025 ml una vez al día |
Semana 3 – 4 (si los efectos adversos son aceptables) | 0,5 mg una vez al día | 0,05 ml una vez al día |
Semana 5 en adelante (si los efectos adversos son aceptables) | 1 mg una vez al día | 0,1 ml una vez al día |
Si la dosis no es suficiente y los efectos adversos son aceptables | 2 mg una vez al día | 0,2 ml una vez al día |
Si los efectos adversos de la dosis inicial de 0,25 mg no son aceptables, se deberá discontinuar el tratamiento.
Después de la dosis inicial, si los efectos adversos de una dosis posterior no son aceptables, se reducirá la dosis al nivel de dosis anterior. Si los efectos adversos de la dosis reducida son aceptables, continuará el aumento de la dosis.
Si los efectos adversos de la dosis de 2 mg no son aceptables, se reducirá a 1 mg y se le seguirá administrando esta dosis.
En niños de 2 a menos de 6años de edadcon insuficiencia renal grave, las dosis recomendadas son las siguientes:
Peso del paciente/semana de tratamiento | Dosis diaria | Volumen que debe inyectarse |
<20kg | ||
Semana 1 en adelante | 0,25 mg una vez al día | 0,025 ml una vez al día |
20-<30kg | ||
Semanas 1-2 | 0,25 mg una vez al día | 0,025 ml una vez al día |
Semana 3 en adelante (si la dosis no es suficiente y los efectos adversos son aceptables) | 0,5 mg una vez al día | 0,05 ml una vez al día |
30-<40kg | ||
Semanas 1-2 | 0,25 mg una vez al día | 0,025 ml una vez al día |
Semanas 3-4 (si la dosis no es suficiente y los efectos adversos son aceptables) | 0,5 mg una vez al día | 0,05 ml una vez al día |
Semana 5 en adelante (si la dosis no es suficiente y los efectos adversos son aceptables) | 1 mg una vez al día | 0,1 ml una vez al día |
≥40kg | ||
Semanas 1-2 | 0,25 mg una vez al día | 0,025 ml una vez al día |
Semanas 3-4 (si la dosis no es suficiente y los efectos adversos son aceptables) | 0,5 mg una vez al día | 0,05 ml una vez al día |
Semanas 5-6 (si la dosis no es suficiente y los efectos adversos son aceptables) | 1 mg una vez al día | 0,1 ml una vez al día |
Semana 7 en adelante (si la dosis no es suficiente y los efectos adversos son aceptables) | 1,5 mg una vez al día | 0,15 ml una vez al día |
Si los efectos adversos de la dosis inicial de 0,25 mg no son aceptables, se debe suspender el tratamiento.
Después de la dosis inicial, si los efectos adversos de una dosis posterior no son aceptables, se reducirá la dosis al nivel de dosis anterior. Si los efectos adversos de la dosis reducida son aceptables, continuará el aumento de la dosis.
Síndrome de Bardet-Biedl
En adultos y niños a partir de los 16 años de edad, las dosis recomendadas son las siguientes:
Semana de tratamiento | Dosis diaria en mg | Volumen que debe inyectarse |
Semanas 1 – 2 | 2 mg una vez al día | 0,2 ml una vez al día |
Semana 3 en adelante (si los efectos adversos son aceptables) | 3 mg una vez al día | 0,3 ml una vez al día |
Si los efectos adversos de la dosis inicial de 2 mg no son aceptables, se reducirá a 1 mg (0,1 ml). Si los efectos adversos de la dosis de 1 mg una vez al día son aceptables, continuará el aumento de la dosis.
Después de la dosis inicial, si los efectos adversos de una dosis posterior no son aceptables, se reducirá la dosis al nivel de dosis anterior. Si los efectos adversos de la dosis reducida son aceptables, continuará el aumento de la dosis.
Si los efectos adversos de la dosis de 3 mg no son aceptables, se reducirá a 2 mg y se le seguirá administrando esta dosis.
En niños de 6 a menos de 16 años de edad, las dosis recomendadas son las siguientes:
Semana de tratamiento | Dosis diaria en mg | Volumen que debe inyectarse |
Semana 1 | 1 mg una vez al día | 0,1 ml una vez al día |
Semana 2 (si los efectos adversos son aceptables) | 2 mg una vez al día | 0,2 ml una vez al día |
Semana 3 en adelante (si los efectos adversos son aceptables) | 3 mg una vez al día | 0,3 ml una vez al día |
Si los efectos adversos de la dosis inicial de 1 mg no son aceptables, se reducirá a 0,5 mg (0,05 ml). Si los efectos adversos de la dosis de 0,5 mg son aceptables, continuará el aumento de la dosis.
Después de la dosis inicial, si los efectos adversos de una dosis posterior no son aceptables, se reducirá la dosis al nivel de dosis anterior.
Si los efectos adversos de la dosis reducida son aceptables, continuará el aumento de la dosis.
Si los efectos adversos de la dosis de 3 mg no son aceptables, se reducirá a 2 mg y se le seguirá administrando esta dosis.
En niños de 2 a <6años de edad, las dosis recomendadas son las siguientes:
Peso del paciente/semana de tratamiento | Dosis diaria | Volumen que debe inyectarse |
<20kg | ||
Semana 1 en adelante | 0,5 mg una vez al día | 0,05 ml una vez al día |
20-<30kg | ||
Semanas 1-2 | 0,5 mg una vez al día | 0,05 ml una vez al día |
Semana 3 en adelante (si la dosis no es suficiente y los efectos adversos son aceptables) | 1 mg una vez al día | 0,1 ml una vez al día |
30-<40kg | ||
Semanas 1-2 | 0,5 mg una vez al día | 0,05 ml una vez al día |
Semanas 3-4 (si la dosis no es suficiente y los efectos adversos son aceptables) | 1 mg una vez al día | 0,1 ml una vez al día |
Semana 5 en adelante (si la dosis no es suficiente y los efectos adversos son aceptables) | 1,5 mg una vez al día | 0,15 ml una vez al día |
≥40kg | ||
Semanas 1-2 | 0,5 mg una vez al día | 0,05 ml una vez al día |
Semanas 3-4 (si la dosis no es suficiente y los efectos adversos son aceptables) | 1 mg una vez al día | 0,1 ml una vez al día |
Semanas 5-6 (si la dosis no es suficiente y los efectos adversos son aceptables) | 1,5 mg una vez al día | 0,15 ml una vez al día |
Semanas 7-8 (si la dosis no es suficiente y los efectos adversos son aceptables) | 2 mg una vez al día | 0,2 ml una vez al día |
Semana 9 en adelante (si la dosis no es suficiente y los efectos adversos son aceptables) | 2,5 mg una vez al día | 0,25 ml una vez al día |
Después de la dosis inicial, si los efectos adversos de una dosis posterior no son aceptables, se reducirá la dosis al nivel de dosis anterior. Si los efectos adversos de la dosis reducida son aceptables, continuará el aumento de la dosis
En pacientes con enfermedad renal leve o moderada, no se necesita ningún cambio en la pauta posológica.
Para adultos y niños de 16 a 17 años de edadcon insuficiencia renal grave, las dosis recomendadas son las siguientes:
Semana de tratamiento | Dosis diaria en mg | Volumen que debe inyectarse |
Semanas 1 – 2 | 0,5 mg una vez al día | 0,05 ml una vez al día |
Semana 3 en adelante (si los efectos adversos son aceptables) | 1 mg una vez al día | 0,1 ml una vez al día |
Si la dosis no es suficiente y los efectos adversos son aceptables | 2 mg una vez al día | 0,2 ml una vez al día |
Si la dosis no es suficiente y los efectos adversos son aceptables | 2,5 mg una vez al día | 0,25 ml una vez al día |
Si la dosis no es suficiente y los efectos adversos son aceptables | 3 mg una vez al día | 0,3 ml una vez al día |
Si los efectos adversos de la dosis inicial de 0,5 mg no son aceptables, se reducirá a 0,25 mg (0,025 ml). Si los efectos adversos de la dosis de 0,25 mg una vez al día son aceptables, continuará el aumento de la dosis.
Después de la dosis inicial, si los efectos adversos de una dosis posterior no son aceptables, se reducirá la dosis al nivel de dosis anterior. Si los efectos adversos de la dosis reducida son aceptables, continuará el aumento de la dosis.
Si los efectos adversos de la dosis de 3 mg no son aceptables, se reducirá a 2,5 mg y se le seguirá administrando esta dosis.
En niños de 6 a menos de 16 años de edadcon insuficiencia renal grave, las dosis recomendadas son las siguientes:
Semana de tratamiento | Dosis diaria en mg | Volumen que debe inyectarse |
Semanas 1 – 2 | 0,25 mg una vez al día | 0,025 ml una vez al día |
Semana 3 – 4 (si los efectos adversos son aceptables) | 0,5 mg una vez al día | 0,05 ml una vez al día |
Semana 5 en adelante (si los efectos adversos son aceptables) | 1 mg una vez al día | 0,1 ml una vez al día |
Si la dosis no es suficiente y los efectos adversos son aceptables | 2 mg una vez al día | 0,2 ml una vez al día |
Si los efectos adversos de la dosis inicial de 0,25 mg no son aceptables, se deberá discontinuar el tratamiento.
Después de la dosis inicial, si los efectos adversos de una dosis posterior no son aceptables, se reducirá la dosis al nivel de dosis anterior.
Si los efectos adversos de la dosis reducida son aceptables, continuará el aumento de la dosis.
Si los efectos adversos de la dosis de 2 mg no son aceptables, se reducirá a 1 mg y se le seguirá administrando esta dosis.
En niños de 2 a menos de 6años de edadcon insuficiencia renal grave, las dosis recomendadas son las siguientes:
Peso del paciente/semana de tratamiento | Dosis diaria | Volumen que debe inyectarse |
<20kg | ||
Semana 1 en adelante | 0,25 mg una vez al día | 0,025 ml una vez al día |
20-<30kg | ||
Semanas 1-2 | 0,25 mg una vez al día | 0,025 ml una vez al día |
Semana 3 en adelante (si la dosis no es suficiente y los efectos adversos son aceptables) | 0,5 mg una vez al día | 0,05 ml una vez al día |
30-<40kg | ||
Semanas 1-2 | 0,25 mg una vez al día | 0,025 ml una vez al día |
Semanas 3-4 (si la dosis no es suficiente y los efectos adversos son aceptables) | 0,5 mg una vez al día | 0,05 ml una vez al día |
Semana 5 en adelante (si la dosis no es suficiente y los efectos adversos son aceptables) | 1 mg una vez al día | 0,1 ml una vez al día |
≥40kg | ||
Semanas 1-2 | 0,25 mg una vez al día | 0,025 ml una vez al día |
Semanas 3-4 (si la dosis no es suficiente y los efectos adversos son aceptables) | 0,5 mg una vez al día | 0,05 ml una vez al día |
Semanas 5-6 (si la dosis no es suficiente y los efectos adversos son aceptables) | 1 mg una vez al día | 0,1 ml una vez al día |
Semana 7 en adelante (si la dosis no es suficiente y los efectos adversos son aceptables) | 1,5 mg una vez al día | 0,15 ml una vez al día |
Si los efectos adversos de la dosis inicial de 0,25 mg no son aceptables, se deberá discontinuar el tratamiento.
Después de la dosis inicial, si los efectos adversos de una dosis posterior no son aceptables, se reducirá la dosis al nivel de la dosis anterior. Si los efectos adversos de la dosis reducida son aceptables, continuará el aumento de la dosis
Su médico debe comprobar regularmente la eficacia del medicamento; el médico puede ajustar la dosis si es necesario. En niños y adolescentes en período de crecimiento, debe supervisarse el impacto en la pérdida de peso y su crecimiento y desarrollo.
Este medicamento está destinado a un uso de larga duración. La interrupción o el uso irregular pueden producir la reaparición o el empeoramiento de los síntomas. Asegúrese de seguir detenidamente la pauta posológica indicada por su médico o farmacéutico.
Cómo inyectar IMCIVREE
IMCIVREE se inyecta en la capa grasa bajo la piel, en el estómago. Su médico, farmacéutico o enfermero le enseñarán cómo hacerlo. Una vez que se sienta cómodo al administrarse la inyección usted mismo o inyectársela a su hijo, podrá hacerlo en casa.
IMCIVREE debe inyectarse al empezar el día para maximizar la reducción del hambre mientras esté despierto. IMCIVREE puede tomarse con independencia de los horarios de las comidas.
Antes de inyectar IMCIVREE, lea detenidamente las instrucciones siguientes.
Paso 1. Prepárese para la inyección
- Tome los artículos que necesite y colóquelos sobre una superficie limpia y plana.
Necesitará los artículos siguientes, que se suministran por separado:
|
|
|
|
|
Lávese las manos con jabón y agua caliente.
- Abra las 2 toallitas con alcohol y la gasa.
Paso 2. Inspeccione el vial
- Compruebe la fecha de caducidad en la etiqueta del vial, que se muestra después de la inscripción «EXP»: MM/AAAA.
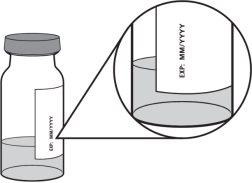
- El líquido debe ser entre transparente y ligeramente amarillento.
- No use IMCIVREE si:
- ha vencido la fecha de caducidad
- el líquido está turbio
- hay partículas flotantes en el vial
- la tapa de plástico de un vial nuevo está rota o ausente
- el vial se ha conservado a temperaturas superiores a 30 ºC.
Paso 3. Prepare el vial
- Antes del uso, deje que el vial alcance la temperatura ambiente. Para hacerlo, retire el vial de la nevera 15 minutos antes de la inyección o haga rodar el vial suavemente entre las palmas de las manos durante 60 segundos.
- No utilice agua caliente, un microondas u otro aparato para calentar el vial.
- No agite el vial.
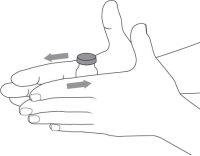
- Si utiliza un nuevo vial, retire la tapa de plástico y deséchela junto con los residuos domésticos.
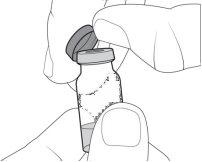
- Limpie la parte superior del tapón gris del vial con una toallita con alcohol. Deseche la toallita con alcohol usada junto con los residuos domésticos.
- No retire el tapón del vial.
Paso 4. Prepare la jeringa
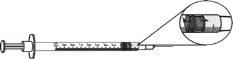
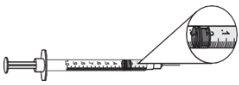
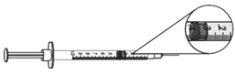
- Para las dosis de 0,25 mg (0,025 ml o 2,5 unidades), usar una jeringa de 0,3 ml con incrementos de 0,5 (media) unidad y una aguja de tamaño 29 a 31 de 6 a 13 mm de longitud, adecuada para inyectar bajo la piel.
dosis de 0,25 mg = 0,025 ml 0 2,5 unidades
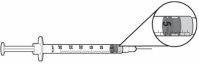
- Para las dosis de 0,5 mg a 3 mg (0,05 ml a 0,3 ml), usar una jeringa de 1 ml con incrementos de 0,01 ml y una aguja de tamaño 28 a 29 de 6 a 13 mm de longitud, adecuada para inyectar bajo la piel.
Dosis de 0,5 mg = 0,05 ml | Dosis de 1 mg = 0,1 ml |
|
|
Dosis de 2 mg = 0,2 ml | Dosis de 3 mg= 0,3 ml |
|
|
- Deje puesta la cubierta protectora de la aguja y tire del émbolo para llenar la jeringa con una cantidad de aire equivalente a la cantidad de medicamento que se utilizará.
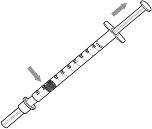
- Retire la cubierta de la aguja de la jeringa. Tire de la cubierta en línea recta y lejos de su cuerpo.
- Coloque el vial en posición vertical sobre una superficie plana. Sujete la jeringa y colóquela directamente sobre el vial. Introduzca la aguja en línea recta en el centro del tapón gris del vial.
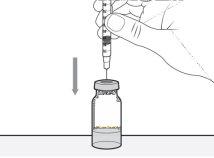
- Presione el émbolo para inyectar el aire de la jeringa en el vial.
- Sin retirar la aguja, gire hacia abajo el vial con cuidado.
- Asegúrese de que el extremo de la aguja esté plenamente dentro del líquido del medicamento y no en el aire por encima del líquido.
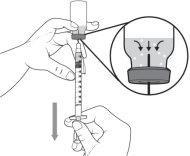
- Tire del émbolo lentamente para llenar la jeringa con la cantidad de medicamento necesaria para su dosis. Al medir la dosis, asegúrese de leer las unidades empezando desde el extremo más cercano al obturador de caucho negro.
- Mantenga la aguja en el vial y compruebe si se han formado grandes burbujas de aire en la jeringa.
Pequeñas burbujas de aire |
| Grandes burbujas de aire |
- Si observa burbujas de aire, deberá eliminarlas de la jeringa. Para eliminarlas:
- Dé unos toques suaves en el lateral de la jeringa con el dedo para que las burbujas de aire se desplacen hacia la parte superior de la jeringa.
- Vuelva a vaciar la jeringa en el vial.
- Siga los pasos anteriores para llenar la jeringa de nuevo. Tire del émbolo más lentamente esta vez y asegúrese de que el extremo de la aguja esté siempre plenamente en el líquido del vial para reducir la probabilidad de que se formen burbujas de aire.
- Cuando no haya grandes burbujas de aire en la jeringa, coloque el vial en posición vertical sobre una superficie dura.
- Sujete el vial con una mano y el cuerpo de la jeringa entre las puntas de los dedos de la otra mano. Tire de la aguja en línea recta hacia arriba y hacia fuera del vial.
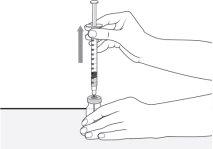
- Coloque la jeringa sobre la superficie dura y asegúrese de que la aguja no toque la superficie.
No vuelva a tapar la jeringa.
Paso 5. Prepare el lugar de inyección
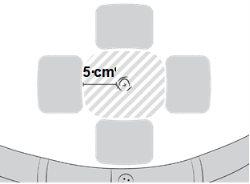
- Elija una zona del vientre para administrar la inyección.
- Cambie el lugar de inyección cada día.
- Asegúrese de que el lugar de inyección se encuentre al menos a 5 cm del ombligo.
- No administre la inyección en una zona enrojecida, hinchada o irritada.
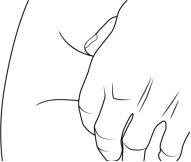
- Limpie el lugar de inyección elegido con la segunda toallita con alcohol con un movimiento circular.
- Deje que la piel se seque durante unos 10 segundos.
- No toque, abanique o sople la zona limpia.
Paso 6. Inyecte IMCIVREE
- Coloque la jeringa entre el dedo pulgar y el dedo índice de la mano con la que escribe.
- Con la otra mano, pellizque suavemente unos 5 cm de piel entre el dedo pulgar y el dedo índice.
Asegúrese de sujetar el pliegue de piel hasta que se haya completado la inyección.
- Sujete el centro de la jeringa a un ángulo de 90º respecto a la piel y empuje la aguja en línea recta hacia el lugar de inyección, asegurándose de que la aguja se introduzca por completo.
- No sujete o empuje el émbolo mientras introduce la aguja.
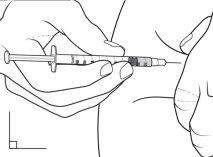
- Sujetando el cuerpo de la jeringa entre el pulgar y el dedo corazón, utilice el dedo índice para empujar lentamente el émbolo e inyectar el medicamento.
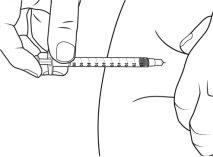
- Cuente hasta 5 después de inyectar IMCIVREE para asegurarse de que todo el medicamento haya salido de la jeringa.
- Suelte el pliegue de piel y retire la aguja.
- Utilice una gasa para presionar suavemente el lugar de inyección y a continuación deséchela junto con los residuos domésticos.
- Deseche la jeringa utilizada en un contenedor específico para objetos cortantes. No la deseche junto con los residuos domésticos.
- Si todavía queda medicamento en el vial, vuelva a colocar el vial en la caja y consérvelo en la nevera o en un lugar seguro a una temperatura inferior a 30 ºC hasta la hora de la siguiente dosis.
Si usa más IMCIVREE del que debe
Si usted o su hijo usan más IMCIVREE del que deben, póngase en contacto con su médico.
Si olvidó usar IMCIVREE
Si se olvidó de inyectar el medicamento, omita la dosis e inyecte la siguiente dosis a la hora habitual. No tome una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con IMCIVREE
Si deja de usar este medicamento, es posible que el hambre regrese y deje de perder peso.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- Marcas o zonas de piel oscurecidas
- Dolor, moretones o inflamación (enrojecimiento o hinchazón) en el lugar de inyección
- Cansancio
- Náuseas o vómitos
- Dolor de cabeza
- Erecciones penianas espontáneas
- Aumento de erecciones penianas
- Neoplasia cutánea
Frecuentes(pueden afectar hasta a 1 de cada 10 personas)
- Sequedad, enrojecimiento o picor de la piel
- Erupción
- Lesiones en la piel
- Pérdida del cabello
- Sensación de debilidad
- Dolor
- Sequedad de boca
- Indigestión
- Diarrea
- Sensación de estreñimiento
- Dolor de estómago
- Acidez
- Sensación de mareo
- Molestias genitales femeninas
- Problemas para dormir
- Sensación de depresión
- Alteración del proceso de excitación sexual
- Deseo sexual aumentado
- Exceso de eosinófilos, un tipo de glóbulo blanco
- Dolor de espalda
- Calambres musculares
- Tos
Poco frecuentes(pueden afectar hasta a 1 de cada 100 personas)
- Enrojecimiento de la piel
- Líneas o manchas en la piel
- Aumento de la sudoración
- Distribución anómala del tejido adiposo
- Erupción pruriginosa
- Piel escamosa
- Sensibilidad al calor o al frío
- Escalofríos
- Sensación de frío
- Sensación de calor
- Alteración del color de las encías
- Hinchazón abdominal
- Aumento de la salivación
- Flatulencia
- Análisis de sangre con niveles elevados de enzimas hepáticas
- Somnolencia
- Migraña
- Pérdida o cambio en el sentido del olfato
- Trastornos del sabor
- Incapacidad femenina para alcanzar o mantener la excitación sexual
- Molestia o sensibilidad genital
- Disminución del deseo sexual
- Trastorno genital femenino
- Dolor menstrual
- Trastorno del sueño
- Pesadillas
- Lunar plano y coloreado en la piel
- Dolores articulares
- Bostezos
- Moqueo
- Dolor en los músculos o huesos
- Dolor en brazos o piernas
- Análisis de sangre con niveles elevados de enzimas musculares
- Decoloración de la parte blanca de los ojos
- Sofocos
- Vértigo
- Trastornos del apetito
- Sensación de sed
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de IMCIVREE
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en el vial. La fecha de caducidad es el último día del mes que se indica.
IMCIVREE debe conservarse en nevera (entre 2 ºC y 8 ºC) hasta la fecha de caducidad indicada en la caja. Alternativamente, IMCIVREE puede conservarse a temperatura ambiente, siempre que no sea superior a 30 ºC, durante un máximo de 30 días o hasta la fecha de caducidad, lo que se produzca primero. Conserve todos los viales (incluso los que haya abierto) en la caja original para protegerlos de la luz. Después de utilizar un vial por primera vez, deséchelo transcurridos 28 días.
No congelar este medicamento.
Si IMCIVREE se expone a temperaturas superiores a 30 ºC, no lo utilice y deséchelo de acuerdo con las directrices locales. No utilice este medicamento si observa partículas flotantes o está turbio.
Utilice siempre una nueva jeringa para cada inyección.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de IMCIVREE
- El principio activo es la setmelanotida. Cada vial multidosis contiene 10 mg de setmelanotida en 1 ml de solución.
Los demás componentes son:
- alcohol bencílico (ver sección 2, «Qué necesita saber antes de empezar a usar IMCIVREE»)
- Sal sódica de N-(carbonil-metoxipolietilenglicol 2000)-1,2-diestearoil-glicero-3- fosfoetanolamina (mPEG-2000-DSPE)
- Carmelosa de sodio (ver sección 2, «Qué necesita saber antes de empezar a usar IMCIVREE»)
- Manitol
- Fenol
- Edetato disódico (ver sección 2, «Qué necesita saber antes de empezar a usar IMCIVREE»)
- Agua para preparaciones inyectables
- Ácido clorhídrico (para ajustar el pH)
- Hidróxido de sodio (para ajustar el pH)
Aspecto del producto y contenido del envase
IMCIVREE es una solución de transparente a ligeramente coloreada.
Este medicamento se presenta en viales de vidrio transparente con un tapón y una tapa y que contienen 1 ml de solución inyectable.
IMCIVREE está disponible en envases que contienen 1 o 10 viales multidosis.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y Responsable de la fabricación
Rhythm Pharmaceuticals Netherlands B.V.
Radarweg 29,
1043NX Amsterdam,
Países Bajos
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a IMCIVREE 10 MG/ML SOLUCION INYECTABLEForma farmacéutica: COMPRIMIDO LIBERACION MODIFICADA, 8 - REVISAR mgPrincipio activo: bupropion and naltrexoneFabricante: Orexigen Therapeutics Ireland LimitedRequiere recetaForma farmacéutica: CAPSULA, 60 mgPrincipio activo: OrlistatFabricante: Haleon Ireland Dungarvan LimitedNo requiere recetaForma farmacéutica: CAPSULA, 60 mgPrincipio activo: OrlistatFabricante: Haleon Ireland Dungarvan LimitedNo requiere receta
Médicos online para IMCIVREE 10 MG/ML SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de IMCIVREE 10 MG/ML SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes