
HOLOCLAR 79000-316000 CELLS/CM2 EQUIVALENT TO LIVING TISSUE

How to use HOLOCLAR 79000-316000 CELLS/CM2 EQUIVALENT TO LIVING TISSUE
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Holoclar 79,000-316,000 cells/cm2equivalent of living tissue.
Human autologous corneal epithelial cells explanted ex vivo, including stem cells.
This medicinal product is subject to additional monitoring, which will allow for the quick identification of new safety information. You can help by reporting any side effects you may get. See the end of section 4 for how to report side effects.
Read all of this leaflet carefully before this medicine is administered to you because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your surgeon.
- If you get any side effects, talk to your surgeon. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Holoclar and what is it used for
- What you need to know before you are given Holoclar
- How Holoclar is administered
- Possible side effects
- Storage of Holoclar
- Contents of the pack and other information
1. What is Holoclar and what is it used for
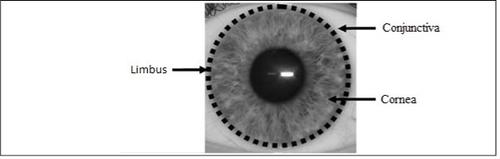
Holoclar is a medicine used as a replacement for damaged corneal cells (the cornea is the transparent layer covering the iris, the colored part in the center of the eye), including limbal cells that normally help to keep your eye healthy.
Holoclar consists of a layer of your cells that have been grown (expanded ex vivo) from a sample of limbal cells taken from your eye during a small surgical procedure called a biopsy. Each preparation of Holoclar is made individually and is for a single treatment, although treatments can be repeated. The cells used to manufacture Holoclar are known as autologous limbal cells:
- Autologousmeans they come from your cells.
- The limbusis a part of the eye. It is the border surrounding the central, colored part (iris) of the eye. The image shows the location of the limbus in your eye.
- The limbus contains limbal cellsthat help maintain the health of the eye, and some of these cells are stem cells, from which new cells can originate. These new cells can replace damaged cells in your eye.
Holoclar is implanted to repair the damaged surface of the eye in adults. When the eye has been severely damaged by physical or chemical burns, many scars can form and the limbus can be affected. Damage to the limbus interferes with the normal healing process, which means the injury to your eye will never completely heal.
By taking healthy limbal cells, a new layer of healthy tissue is produced in the laboratory and grown on a layer of fibrin, a type of protein that serves as a scaffold. This tissue layer is then implanted by the surgeon into the damaged cornea, helping your eye to heal naturally.
2. What you need to know before you are given Holoclar
Holoclar must not be implanted:
- if you are allergic to any of the components of this medicine (listed in section 6) or to bovine serum and mouse cells.
Warnings and precautions
Talk to your surgeon before Holoclar is implanted.
Holoclar is prepared individually from your cells, only for your personal use, and must not be used by anyone else.
If you have an eye infection or redness (inflammation) of the eyes, your treatment should be postponed until you recover.
When Holoclar is manufactured, two components of animal origin are used. One is fetal bovine serum, which comes from cows and is used to grow your cells. The other component is a special type of inactivated mouse cells used to stimulate the growth of your limbal cells. If you are allergic to any of these components, you must not be given this medicine (see above, under “Holoclar must not be implanted”).
If you have any of the following problems in your eyes, they must be treated before using this medicine:
- Irregular eyelids
- Conjunctival scarring with lesions on the surface in contact with the inside of the eyelids (shortening of the fornix)
- Lack of feeling in the eye (corneal or conjunctival anesthesia or hypoesthesia)
- Growth of the conjunctiva over the cornea (pterigium)
- Severe dry eye
Other cases where Holoclar cannot be used
Although the surgeon has already taken a small sample of limbal cells (a biopsy) needed to manufacture the medicine, you may not meet the requirements for treatment with Holoclar. This can happen if your biopsy is not of sufficient quality to manufacture Holoclar, if it is impossible to grow the cells in the laboratory, or if the cells do not meet all quality requirements after being grown. Your surgeon will inform you about this.
Children and adolescents
To date, only a limited number of children have been treated, so it is not known whether the medicine is safe for use in children or how effective it is.
Kidney and liver problems
Talk to your surgeon before starting treatment if you have any kidney or liver disease.
Using Holoclar with other medicines
Some eye drops contain a preservative called “benzalkonium chloride”. This component can damage the cells that make up Holoclar. Do not use eye drops that contain benzalkonium chloride or other preservatives. Ask your doctor or pharmacist for more information.
Pregnancy and breastfeeding
If you are pregnant, think you may be pregnant, or are breastfeeding, you should postpone treatment with this medicine.
Driving and using machines
Holoclar is administered through eye surgery, and this will affect your ability to drive and use machines. Therefore, you should not drive or use machines after Holoclar has been implanted in your eye until your surgeon tells you it is safe to do so. Follow their instructions carefully.
3. How Holoclar is administered
Holoclar can only be prescribed and administered by an eye surgeon in a hospital.
Treatment with Holoclar is a two-stage procedure.
Visit 1: Biopsy
During the first visit, the surgeon will perform a biopsy, which means removing a very small amount of tissue containing limbal cells (from your eye). Before the biopsy, the surgeon will give you eye drops to anesthetize your eye and perform the surgical procedure. The biopsy will be used to manufacture Holoclar. After the biopsy, your surgeon will prescribe antibiotic treatment to reduce the risk of infection.
The production of Holoclar will take several weeks.
Visit 2: Holoclar implantation
During the second visit, the surgeon will:
- Anesthetize your eye
- Remove the scarred surface of the cornea
- Replace it with Holoclar
On the day of surgery, the surgeon will anesthetize your eye and then sew the edge of your new cornea with stitches to ensure Holoclar stays in place. Your eyelid will be kept closed with a patch for three days, and your eye will be bandaged for a period of 10 to 15 days after implantation.
After surgery, you will be prescribed treatment for complete recovery: antibiotics to reduce the risk of infection and steroids to reduce inflammation and irritation. It is very important that you use all the medicines prescribed by your surgeon, as otherwise, the implantation of Holoclar may not work.
Read the package leaflets of the individual medicines prescribed to you for more information about them.
If you have any doubts about treatment with Holoclar, ask your surgeon.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Most side effects are eye-related, some of which are caused directly by the surgery. Most side effects are mild and disappear in the weeks following surgery.
The most serious side effects are problems with the cornea (erosion) and corneal perforation, which may occur during the 3 months following Holoclar implantation. In this case, contact your surgeon.
Very common side effects: may affect more than 1 in 10 people
- Inflammation of the eyelids (blepharitis)
Common side effects: may affect up to 1 in 10 people
- Bleeding around the site where Holoclar was implanted
- Corneal problems (erosion)
- Increased intraocular pressure (glaucoma)
- Eye pain
- Corneal inflammation
Uncommon side effects: may affect up to 1 in 100 people
- Eye disorders: sticky eyelids, red eyes, eye swelling, corneal perforation, and eye irritation
- Sensitivity to light
- Growth around the implant (metaplasia)
- Corneal infection
- Breaking of stitches
- Fainting
- Bleeding from the eyelid skin
Reporting of side effects
If you experience any side effects, talk to your surgeon, even if they are not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Monitoring System for Human Use https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Holoclar
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label.
Do not store above 25°C or below 15°C.
Do not refrigerate or freeze.
Keep Holoclar in the steel container, in the plastic bag, until surgery. This is to protect it from bacterial contamination.
Holoclar must not be irradiated or sterilized.
Since this medicine will be used during your surgery, healthcare professionals are responsible for the proper storage of the medicine before and during its use, as well as its proper disposal.
6. Contents of the pack and other information
Composition of Holoclar
- The active ingredient consists of between 300,000 and 1,200,000 living eye cells from your biopsy, of which an average of 3.5% are stem cells. Each square centimeter of Holoclar contains between 79,000 and 316,000 cells.
- It contains two excipients, one of which is fibrin (a transparent support layer to keep Holoclar intact), the other is a liquid containing amino acids, vitamins, salts, and carbohydrates to store the cells in the vial called modified Eagle's medium by Dulbecco, DMEM, supplemented with L-glutamine.
Appearance of the product and pack contents
Holoclar is a layer of cells to be implanted in your eye. The cells are kept alive in a small sterile container. The medicine is covered with several layers of packaging designed to protect the medicine from bacteria and ensure that Holoclar remains at a constant temperature for 36 hours if stored at room temperature.
Each package contains a single treatment dose that is large enough to cover your cornea.
Marketing authorization holder and manufacturer
Holostem Terapie Avanzate s.r.l.
Via Glauco Gottardi 100, 41125 Modena (Italy)
Phone: +39 059 2058070
Fax: +39 059 2058115
Date of last revision of this leaflet
This medicine has been authorized with a “conditional approval”. This type of approval means that more information on this medicine is expected.
The European Medicines Agency will review new information on this medicine at least once a year, and this leaflet will be updated as necessary.
Other sources of information
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to HOLOCLAR 79000-316000 CELLS/CM2 EQUIVALENT TO LIVING TISSUEDosage form: EYEDROP, 5.5 mg sodium chloride; 3 mg hypromellose/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Alcon Healthcare S.A.Prescription not requiredDosage form: EYEDROP, 3.2 mg/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Bausch & Lomb S.A.Prescription not requiredDosage form: EYE DROP, 3.2 mg/mlActive substance: artificial tears and other indifferent preparationsManufacturer: Bausch & Lomb S.A.Prescription not required
Online doctors for HOLOCLAR 79000-316000 CELLS/CM2 EQUIVALENT TO LIVING TISSUE
Discuss questions about HOLOCLAR 79000-316000 CELLS/CM2 EQUIVALENT TO LIVING TISSUE, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions










