
HIDROFEROL 0,266 mg SOLUCION ORAL

Cómo usar HIDROFEROL 0,266 mg SOLUCION ORAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
- Introducción
- Qué es Hidroferol 0,266 mg solución oral y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Hidroferol 0,266 mg solución oral
- Cómo tomar Hidroferol 0,266 mg solución oral
- Posibles efectos adversos
- Conservación de Hidroferol 0,266 mg solución oral
- Contenido del envase e información adicional
Introducción
Prospecto: información para el usuario
Hidroferol 0,266 mg solución oral
calcifediol monohidrato
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque
contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido delprospecto
- Qué es Hidroferol 0,266 mg solución oral y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Hidroferol 0,266 mg solución oral
- Cómo tomar Hidroferol 0,266 mg solución oral
- Posibles efectos adversos
- Conservación de Hidroferol 0,266 mg solución oral
- Contenido del envase e información adicional
1. Qué es Hidroferol 0,266 mg solución oral y para qué se utiliza
Contiene una forma de vitamina D, calcifediol, que se usa para tratar la deficiencia de vitamina D y los problemas que de ella se derivan. La vitamina D interviene en el organismo humano, entre otras acciones, aumentando la absorción del calcio.
Hidroferol 0,266 mg solución oral está indicado para el tratamiento de la deficiencia de vitamina D en adultos y para prevenir la deficiencia de vitamina D en adultos con riesgos identificados como en pacientes con síndrome de malabsorción, enfermedad renal crónica – enfermedad mineral ósea (ERC-EMO) o cualquier otro riesgo identificado.
También se utiliza para tratar la pérdida ósea (osteoporosis), junto con otros medicamentos, en pacientes con deficiencia de vitamina D o con riesgo de deficiencia de vitamina D.
2. Qué necesita saber antes de empezar a tomar Hidroferol 0,266 mg solución oral
No tomeHidroferol0,266mg solución oral:
- si es alérgico al calcifediol o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si tiene hipercalcemia (niveles elevados de calcio en sangre) o hipercalciuria (niveles elevados de calcio en orina).
- si padece formación de piedras (cálculos) de calcio.
- si tiene hipervitaminosis D (exceso de vitamina D en el organismo).
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a tomar Hidroferol 0,266 mg solución oral.
- No debe sobrepasar las cantidades diarias recomendadas de suplementos con vitamina D, como este medicamento, debido a que se podría producir una intoxicación. (ver sección 3 apartado Si toma másHidroferol 0,266mg solución oralde lo que debe).
- Mientras esté tomando este medicamento o antes de empezar, es posible que su médico le indique realizarse análisis de sangre u orina para controlar sus niveles de calcio, fósforo y otros parámetros.
- Los pacientes con enfermedad del riñón requieren una especial precaución y deben ser especialmente vigilados por su médico, con la realización de análisis periódicos.
- Los pacientes con enfermedad del corazón requieren una especial precaución y deben ser supervisados frecuentemente por su médico para el control del calcio en sangre, especialmente los que estén en tratamiento con glucósidos cardiacos (ver en esta sección, apartado Otros medicamentos eHidroferol 0,266mgsolución oral).
- Si padece hipoparatiroidismo (función insuficiente de la hormona paratiroidea) podría disminuir la acción de este medicamento.
- Si padece de formación de cálculos (piedras) en el riñón, el médico debe controlar sus niveles de calcio en sangre.
- Los enfermos con una inmovilización prolongada podrían necesitar dosis menores de este medicamento.
- Los pacientes con sarcoidosis (enfermedad con nódulos, generalmente en la piel), con tuberculosis o con otras enfermedades con nódulos, deben tener especial precaución con este medicamento, ya que tienen más riesgo de sufrir efectos adversos a dosis inferiores a las recomendadas. Deberán realizarse análisis periódicos para controlar los niveles de calcio en sangre y orina.
- Interferencias con pruebas analíticas: si le van a realizar alguna prueba diagnóstica (incluidos análisis de sangre, orina, pruebas cutáneas que utilizan alergenos, etc.) comunique al médico que está tomando este medicamento, ya que puede alterar los resultados. Por ejemplo, en alguna prueba de determinación de colesterol.
Niños y adolescentes
No se ha establecido la seguridad y eficacia de calcifediol en niños y adolescentes menores de 18 años. No existen datos disponibles.
Para uso en niños existe en el mercado el medicamento Hidroferol 0,1mg/ml gotas orales en solución, con menor concentración de dosis.
Otros medicamentos e Hidroferol 0,266mg solución oral
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento, incluso los adquiridos sin receta.
Algunos medicamentos pueden afectar a la forma en la que actúa este medicamento. Por otra parte, su principio activo, calcifediol monohidrato, puede afectar a la eficacia de otros medicamentos que se tomen al mismo tiempo.
Por tanto, se pueden producir interacciones con los siguientes medicamentos:
- Medicamentos utilizados en el tratamiento de la epilepsia (como fenitoína, fenobarbital y primidona) y otros medicamentos inductores enzimáticos (favorecen la disminución del efecto de Hidroferol).
- Medicamentos para el corazón y/o para la hipertensión arterial como los glucósidos cardiacos, los diuréticos tiazídicos o verapamilo.
- Colestiramina, colestipol (para el colesterol), orlistat (para la obesidad): se debe separar la toma de estos medicamentos y calcifediol al menos 2 horas.
- Aceite mineral o parafina (laxantes): se recomienda utilizar otro tipo de laxante o separar las tomas de ambos medicamentos.
- Algunos antibióticos (como penicilina, neomicina y cloranfenicol).
- Sales de magnesio.
- Otros productos con Vitamina D.
- Suplementos de calcio.
- Corticosteroides (medicamentos antiinflamatorios).
Toma de Hidroferol 0,266mg solución oral con alimentos y bebidas
Algunos alimentos y bebidas están suplementados con vitamina D. Esto debe tenerse en cuenta ya que los efectos de estos alimentos se podrían sumar a los efectos de este medicamento y resultar excesivos.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No tome este medicamento durante el embarazo.
No tome este medicamento mientras está amamantando a su hijo.
Conducción y uso de máquinas
Este medicamento no afecta la capacidad de conducir vehículos ni de manejar máquinas.
3. Cómo tomar Hidroferol 0,266 mg solución oral
La ampolla de Hidroferol 0,266 mg solución oral es EXCLUSIVAMENTE para administración por VÍA ORAL.
Hidroferol 0,266 mg solución oral NO se debe administrar por vía intramuscular y/o intravenosa.
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
NO tome Hidroferol 0,266 mg solución oral DIARIAMENTE.No tome más cantidad de medicamento ni lo tome con más frecuencia de lo indicado por su médico (semanal, quincenal o mensual). Si usted lo hace, puede aumentar la posibilidad de una sobredosis.
Las dosis recomendadas son:
- Tratamiento de la deficiencia de vitamina D y prevención de la deficiencia de vitamina D en pacientes con riesgos identificados: una ampolla bebible (0,266 mg de calcifediol monohidrato), una vez al mes.
- Como complemento a la medicación especifica de la pérdida de masa ósea: una ampolla bebible (0,266 mg de calcifediol monohidrato), una vez al mes.
Existen poblaciones de alto riesgo de deficiencia de vitamina D en las que puede ser necesario administrar dosis superiores. Tras el correspondiente análisis del grado de deficiencia, el médico puede considerar la dosis de una ampolla cada dos semanas o todas las semanas.
Su médico deberá controlar sus niveles de calcio y Vitamina D periódicamente, normalmente antes de empezar el tratamiento y a los 3-4 meses una vez iniciado el tratamiento. Según la indicación, las dosis se reducirán o espaciarán más en el tiempo cuando mejoren los síntomas o se haya corregido la deficiencia de vitamina D.
Vía oral.
El contenido de la ampolla se puede tomar solo o se puede diluir en un poco de agua, leche o zumo.
Las instrucciones de apertura y uso de las ampollas son como sigue:
Agitar la ampolla antes de usar.
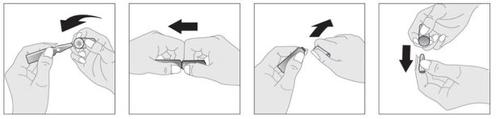
Introducir la cabeza de la ampolla de vidrio en el cilindro facilitador de la apertura. Sostener el cuerpo de la ampolla entre el pulgar y el dedo índice con el punto blanco hacia arriba.
Colocar el dedo índice de la otra mano por debajo del cillindro que cubre la cabeza de la ampolla, y el pulgar por encima del mismo tal y como indica la figura, de manera que los dedos índice de las dos manos se toquen entre sí por debajo de la ampolla.
Mantener la mano que sujeta la ampolla sin mover y presionar la cabeza con el pulgar hacia abajo para abrir la ampolla con un movimiento seco.
Una vez abierta la ampolla, retirar la cabeza de la ampolla del cilindro para su posterior reutilización.
Si estima que la acción de este medicamento es demasiado fuerte o débil, comuníqueselo a su médico o farmacéutico.
Si toma másHidroferol0,266 mg solución oral del que debe
Si toma más cantidad de este medicamento que la dosis indicada por su médico (sobredosis) y/o durante tiempo prolongado, podría aparecer hipercalcemia (elevados niveles de calcio en sangre) y fosfatos en sangre y orina, llevando a una posible insuficiencia renal. Algunos síntomas de la toxicidad pueden aparecer pronto y otros cuando ha transcurrido un tiempo.
Entre los síntomas iniciales están: debilidad, fatiga, dolor de cabeza, falta de apetito, sequedad de boca, trastornos digestivos como vómitos, calambres en el abdomen, estreñimiento o diarrea, aumento de la sed; aumento de las micciones (orinas), dolor muscular.
Entre los síntomas que se manifiestan después de un tiempo podrá padecer: picores, pérdida de peso, retraso en el crecimiento en niños, trastornos del riñón, intolerancia a la luz solar, conjuntivitis, aumento del colesterol, de las transaminasas, inflamación del páncreas, calcificación (depósito de sales de calcio) en los vasos sanguíneos y en otros tejidos como los tendones y los músculos, aumento de la tensión arterial, trastornos mentales, latidos irregulares del corazón.
Los síntomas de la sobredosis suelen mejorar o desaparecer al interrumpir el tratamiento, pero si la intoxicación es grave podría provocar un fallo del riñón o del corazón.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o acuda a un centro médico, o llame al Servicio de Información Toxicológica, Teléfono 91 562 04 20, indicando el medicamento y la cantidad ingerida (o lleve el medicamento con usted).
Si olvidó tomarHidroferol0,266 mg solución oral
No tome una dosis doble para compensar las dosis olvidadas.
Tome la dosis olvidada lo más pronto posible; luego vuelva a su horario regular de dosificación.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Se pueden producir efectos adversos si se toman dosis excesivas o con una frecuencia superior a lo indicado por el médico, que pueden producir hipercalcemia (niveles elevados de calcio en sangre) e hipercalciuria (niveles elevados de calcio en orina); ver sección 3 para la descripción de los síntomas.
Otros efectos adversos incluyen reacciones alérgicas como picor, hinchazón local, dificultad para respirar y enrojecimiento de la piel.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Hidroferol 0,266 mg solución oral
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
No requiere condiciones especiales de conservación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición deHidroferol0,266 mg solución oral
- El principio activo es calcifediol monohidrato. Cada ampolla contiene 0,266 mg de calcifediol monohidrato.
- Los demás componentes (excipientes) son: triglicéridos de cadena media y acetato de alfa-tocoferol.
Aspecto del producto y contenido del envase
Hidroferol 0,266 mg es una solución oral; es un líquido transparente, incoloro o ligeramente amarillento, viscoso y libre de impurezas visibles; se presenta en ampollas de vidrio.
Cada envase contiene 10 ampollas bebibles.
Otras presentaciones de Hidroferol:
Hidroferol 0,1 mg/ml gotas orales en solución.
Hidroferol 0,266 mg cápsulas blandas
Hidroferol Choque 3 mg solución oral.
Titular de la autorización de comercialización
Faes Farma, S.A.
Autonomia Etorbidea, 10
48940 Leioa (Bizkaia)
España
Responsabledelafabricación
Faes Farma Portugal, S.A.Rua Elias Garcia, 28
Amadora-P-2700
Portugal
Fecha de la última revisión de este prospecto: noviembre 2023
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia10.32 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a HIDROFEROL 0,266 mg SOLUCION ORALForma farmacéutica: CAPSULA, 0,266 mgPrincipio activo: calcifediolFabricante: Laboratorios Normon S.A.Requiere recetaForma farmacéutica: CAPSULA, 0,266 mgPrincipio activo: calcifediolFabricante: Laboratorios Normon S.A.Requiere recetaForma farmacéutica: SOLUCION/SUSPENSION GOTAS ORALES, 0,1 mgPrincipio activo: calcifediolFabricante: Faes Farma S.A.Requiere receta
Médicos online para HIDROFEROL 0,266 mg SOLUCION ORAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de HIDROFEROL 0,266 mg SOLUCION ORAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















