
FLUENZ SUSPENSION PARA PULVERIZACION NASAL

Cómo usar FLUENZ SUSPENSION PARA PULVERIZACION NASAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
Fluenz suspensión para pulverización nasal
Vacuna frente a la gripe (viva, nasal)
Lea todo el prospecto detenidamente antes de administrar la vacuna, porque contiene información importante para usted o para su hijo.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, enfermero o farmacéutico.
- Esta vacuna se le ha recetado solamente a usted o a su hijo y no debe dársela a otras personas.
- Si experimenta efectos adversos, consulte a su médico, enfermero o farmacéutico. Esto incluye cualquier efecto adverso posible no mencionado en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es Fluenz y para qué se utiliza
- Qué necesita saber antes de que le administren Fluenz
- Cómo se administra Fluenz
- Posibles efectos adversos
- Conservación de Fluenz
- Contenido del envase e información adicional
1. Qué es Fluenz y para qué se utiliza
Fluenz es una vacuna para prevenir la gripe. Se utiliza en niños y adolescentes de más de 2 años y menos de 18 años. Fluenz le ayudará a protegerse de las cepas de virus contenidas en la vacuna y otras cepas estrechamente relacionadas con ellas.
Cómo actúa Fluenz
Cuando se administra la vacuna a una persona, el sistema inmunitario (el sistema de defensa natural del organismo) produce su propia protección frente al virus de la gripe. Ninguno de los componentes de la vacuna puede provocar la gripe.
Los virus de la vacuna Fluenz se cultivan en huevos de gallina. Cada año, la vacuna actúa frente a tres cepas de la gripe, siguiendo las recomendaciones anuales de la Organización Mundial de la Salud.
2. Qué necesita saber antes de que le administren Fluenz
No se le administrará Fluenz:
- si es alérgicoa los principios activos, la gentamicina (trazas de residuo del proceso de fabricación), o a alguno de los demás componentes de esta vacuna enumerados en la sección 6 .
- si alguna vez ha tenido una reacción alérgica grave a los huevos o a las proteínas del huevo. Para conocer los signos de las reacciones alérgicas, ver la sección 4.
- si padece un trastorno de la sangreo un cáncerque afecte al sistema inmunitario.
- si su médico le ha dichoque tiene usted el sistema inmunitario debilitadoa consecuencia de una enfermedad, medicamento u otro tratamiento (como leucemias agudas y crónicas, linfoma, infección sintomática por VIH, inmunodeficiencias celulares y dosis altas de corticosteroides).
- si ya está tomandoácido acetilsalicílico(una sustancia presente en muchos medicamentos utilizados para aliviar el dolor y bajar la fiebre).Esto se debe al riesgo de una enfermedad muy rara pero grave (el síndrome de Reye).
Si se cumple alguna de estas condiciones, informe a su médico, enfermero o farmacéutico.
Advertencias y precauciones
Su médico o enfermero se asegurarán de que dispone de tratamiento médico adecuado y supervisión en caso de que se produzca una reacción anafiláctica poco frecuente (una reacción alérgica muy grave con síntomas como dificultad para respirar, mareo, pulso débil y rápido y erupción cutánea) tras la administración.
Informe a su médico, enfermero o farmacéutico antes de la vacunación:
- si padece asma graveo actualmente sibilancias.
- si padece una enfermedad aguda grave asociada a fiebre o infección, o si tiene obstrucción nasal. Su médico puede decidir retrasar la vacunación hasta que haya desaparecido la fiebre o la obstrucción nasal.
- si está en contacto estrecho con alguien que tiene el sistema inmunitario fuertemente debilitado(por ejemplo, un paciente trasplantado de médula ósea que necesita aislamiento).
- si tiene algún defecto congénito que afecte a los huesos y tejidos del cráneo y la cara, que no haya sido corregido quirúrgicamente.
Si se cumple alguna de estas condiciones, informe a su médico, enfermero o farmacéutico antes de la vacunación. Él o ella decidirá si Fluenz es adecuado para usted.
Niños menores de2años de edad
Los niños menores de 2 años no deben recibir esta vacuna debido al riesgo de efectos adversos.
Otros medicamentos, otras vacunas y Fluenz
Informe a su médico, enfermero o farmacéutico si la persona vacunada está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento, incluyendo medicamentos que no requieran prescripción.
- No administreácido acetilsalicílico(una sustancia presente en muchos medicamentos utilizados para aliviar el dolor y bajar la fiebre) a niñosdurante 4 semanas después de la vacunación con Fluenz a menos que su médico, enfermero o farmacéutico le indique lo contrario. Esto se debe al riesgo de sufrir síndrome de Reye, una enfermedad muy rara, pero grave, que puede perjudicar al cerebro y al hígado.
- Se recomienda no administrar Fluenzal mismo tiempo que medicamentos antiviralescomo oseltamivir y zanamivir. Esto se debe a que la vacuna podría perder eficacia.
Su médico, enfermero o farmacéutico decidirán si se puede administrar Fluenz al mismo tiempo que otras vacunas.
Embarazo y lactancia
- Si está embarazada, cree que podría estar embarazada, tiene intención de quedarse embarazada o está en periodo de lactancia, consulte a su médico, enfermero o farmacéuticoantes de utilizar esta vacuna. Fluenz no se recomiendapara mujeres embarazadas o en periodo de lactancia.
Conducción y uso de máquinas
- La influencia de Fluenz sobre la capacidad para conducir y utilizar máquinas es nula o insignificante.
3. Cómo se administra Fluenz
Fluenz solo debe utilizarse en pulverización nasal.
Fluenz no debe inyectarse.
Fluenz se administrará como una pulverización en cada fosa nasal. Puede respirar con normalidad mientras se le administra Fluenz. No hace falta que inhale ni aspire activamente.
Posología
La dosis recomendadapara niños y adolescentes es de 0,2 ml de Fluenz, administrados a razón de 0,1 ml en cada fosa nasal. Los niños de 2 a 8años de edad que no han sido vacunados antes frente a la griperecibirán una segunda dosis de seguimiento tras un intervalo mínimo de 4 semanas. Siga las instrucciones de su médico, enfermero o farmacéutico acerca de si su hijo debe acudir para la segunda dosis y cuándo.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, enfermero o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, esta vacuna puede producir efectos adversos, aunque no todas las personas los sufran.
Consulte a su médico, enfermero o farmacéutico si desea más información acerca de los posibles efectos adversos de Fluenz.
Algunos efectos adversos pueden ser graves.
Muy raros(pueden afectar hasta 1 de cada 10 000 personas)
- reacciones alérgicas graves: entre los signos de reacción alérgica pueden figurar dificultad para respirar e hinchazón de la cara o de la lengua.
Informe a su médico de inmediato o busque atención sanitaria urgente si nota alguno de estos síntomas.
Otros posibles efectos adversos de Fluenz
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- nariz congestionada o con mocos
- disminución del apetito
- malestar general
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
- fiebre
- dolores musculares
- dolor de cabeza
Poco frecuentes(pueden afectar hasta 1 de cada 100 personas)
- erupción cutánea
- hemorragia nasal
- reacciones alérgicas
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Fluenz
Mantener esta vacuna fuera de la vista y del alcance de los niños.
No utilice esta vacuna después de la fecha de caducidad que aparece en la etiqueta del aplicador y en el cartonaje después de las letras EXP/CAD.
Conservar en nevera (entre 2 ºC y 8 ºC). No congelar.
Conserve el aplicador nasal en el embalaje exterior para protegerlo de la luz.
Antes del uso, la vacuna se puede sacar de la nevera una vez durante un periodo máximo de 12 horas a una temperatura de hasta 25 °C. Si no se ha utilizado tras este periodo de 12 horas, la vacuna se debe desechar.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Fluenz
Los principios activos son:
Virus influenza reagrupado* (vivo atenuado) de las siguientes tres cepas**:
Cepa similar a A/Victoria/4897/2022 (H1N1)pdm09
(A/Norway/31694/2022, MEDI 369815) 107,0±0,5 UFF***
Cepa similar a A/Croatia/10136RV/2023 (H3N2)
(A/Perth/722/2024, MEDI 392611) 107,0±0,5 UFF***
Cepa similar a B/Austria/1359417/2021
(B/Austria/1359417/2021, MEDI 355292) 107,0±0,5 UFF***
.......................................................................................................por dosis de 0,2 ml
- Multiplicado en huevos de gallina fertilizados de gallineros sanos.
** Producidas en células VERO por tecnología genética inversa. Este producto contiene organismos modificados genéticamente (OMG).
*** Unidades de Focos Fluorescentes
Esta vacuna cumple con la recomendación de la OMS (Organización Mundial de la Salud, Hemisferio Norte) y la decisión de la UE para la temporada 2025/2026.
La vacuna puede contener trazas de las siguientes sustancias: proteínas de huevo (p. ej, ovoalbúmina) y gentamicina. Los demás componentes son sacarosa, hidrógenofosfato de potasio, dihidrógenofosfato de potasio, gelatina, clorhidrato de arginina, monohidrato de glutamato monosódico y agua para inyección.
Aspecto de Fluenz y contenido del envase
Esta vacuna se presenta en suspensión para pulverización nasal (0,2 ml) en un aplicador nasal de un solo uso (de vidrio Tipo 1) en un tamaño de envase de 1 y 10 aplicadores nasales. Puede que solamente estén disponibles en su país algunos tamaños de envases.
La suspensión es de incolora a amarilla clara, de transparente a ligeramente turbia. Puede presentar pequeñas partículas blancas.
Titular de la autorización de comercialización
AstraZeneca AB,
SE-151 85
Södertälje
Suecia
Responsable de la fabricación
AstraZeneca Nijmegen B.V.,
Lagelandseweg 78
Nijmegen, 6545CG
Países Bajos
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien AstraZeneca S.A./N.V. Tel: +32 2 370 48 11 | Lietuva UAB AstraZeneca Lietuva Tel: +370 5 2660550 |
| Luxembourg/Luxemburg AstraZeneca S.A./N.V. Tél/Tel: +32 2 370 48 11 |
Ceská republika AstraZeneca Czech Republic s.r.o. Tel: +420 222 807 111 | Magyarország AstraZeneca Kft. Tel.: +36 1 883 6500 |
Danmark AstraZeneca A/S Tlf: +45 43 66 64 62 | Malta Associated Drug Co. Ltd Tel: +356 2277 8000 |
Deutschland AstraZeneca GmbH Tel: +49 40 809034100 | Nederland AstraZeneca BV Tel: +31 85 808 9900 |
Eesti AstraZeneca Tel: +372 6549 600 | Norge AstraZeneca AS Tlf: +47 21 00 64 00 |
Ελλ?δα AstraZeneca A.E. Τηλ: +30 210 6871500 | Österreich AstraZeneca Österreich GmbH Tel: +43 1 711 31 0 |
España AstraZeneca Farmacéutica Spain, S.A. Tel: +34 91 301 91 00 | Polska AstraZeneca Pharma Poland Sp. z o.o. Tel.: +48 22 245 73 00 |
France AstraZeneca Tél: +33 1 41 29 40 00 | Portugal AstraZeneca Produtos Farmacêuticos, Lda. Tel: +351 21 434 61 00 |
Hrvatska AstraZeneca d.o.o. Tel: +385 1 4628 000 | România AstraZeneca Pharma SRL Tel: +40 21 317 60 41 |
Ireland AstraZeneca Pharmaceuticals (Ireland) DAC Tel: +353 1609 7100 | Slovenija AstraZeneca UK Limited Tel: +386 1 51 35 600 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika AstraZeneca AB, o.z. Tel: +421 2 5737 7777 |
Italia AstraZeneca S.p.A. Tel: +39 02 00704500 | Suomi/Finland AstraZeneca Oy Puh/Tel: +358 10 23 010 |
Κ?προς Αλ?κτωρ Φαρμακευτικ? Λτδ Τηλ: +357 22490305 | Sverige AstraZeneca AB Tel: +46 8 553 26 000 |
Latvija SIA AstraZeneca Latvija Tel: +371 67377100 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos https://www.ema.europa.eu.
‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑‑
Instrucciones para profesionales sanitarios
Esta información está destinada únicamente a profesionales del sector sanitario:
Fluenz es para un uso por vía nasal únicamente.
- No utilizar con una aguja.No inyectar.
- No utilizar Fluenz si la fecha de caducidad ha expirado o si el aplicador está dañado, por ejemplo si el émbolo está suelto o desplazado del aplicador nasal o si hay algún signo de pérdida de contenido.
- Revise la apariencia de la vacuna antes de su administración. La suspensión debe ser de incolora a amarillo pálido, de transparente a opalescente. Puede presentar pequeñas partículas blancas.
- Fluenz se administra como dosis dividida en ambas fosas nasales tal y como se describe a continuación (ver sección 3).
- Tras administrar la mitad de la dosis en una fosa nasal, administrar la otra mitad de la dosis en la otra fosa nasal inmediatamente o poco después.
- El paciente puede respirar normalmente mientras se administra la vacuna; no hace falta inhalar ni aspirar activamente por la nariz.
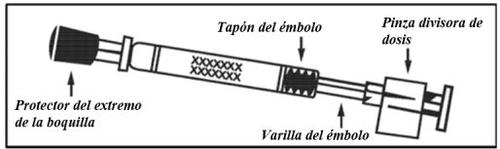
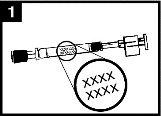
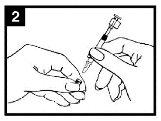
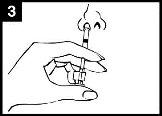
Comprobar la fecha decaducidad El producto no debe utilizarse después de la fecha indicada en la etiqueta del aplicador. | Preparar el aplicador Retirar el protector del extremo de la boquilla. No retirar la pinza divisora de dosis que hay en el otro extremo del aplicador. | Colocar el aplicador Con el paciente en posición vertical, colocar el extremo dentro de la fosa nasal para garantizar que Fluenz se administra en la nariz. |
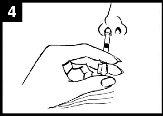

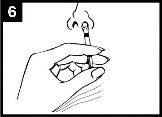
Presionar el émbolo Con un solo movimiento, presionar el émbolo lo más rápidamente posiblehasta que la pinza divisora de dosis impida continuar. | Retirar la pinza divisora de dosis Para administrar en la otra fosa nasal, pinzar y retirar la pinza divisora de dosis del émbolo. | Pulverizar en la otra fosa nasal Colocar el extremo inmediatamente dentro de la otra fosa nasaly, con un solo movimiento, presionar el émbolo lo más rápidamente posiblepara administrar el resto de la vacuna. |
Ver la sección5para obtener información acerca de la conservación y eliminación
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a FLUENZ SUSPENSION PARA PULVERIZACION NASALForma farmacéutica: PRODUCTO USO NASAL, 7,0 ± 0,5 LOG10 FFUPrincipio activo: influenza, live attenuatedFabricante: Astrazeneca AbRequiere recetaForma farmacéutica: INYECTABLE, 3,75 microgramosPrincipio activo: influenza, inactivated, split virus or surface antigenFabricante: Glaxosmithkline BiologicalsRequiere recetaForma farmacéutica: INYECTABLE, 0,5 mlPrincipio activo: influenza, inactivated, split virus or surface antigenFabricante: Sanofi Winthrop IndustrieRequiere receta
Médicos online para FLUENZ SUSPENSION PARA PULVERIZACION NASAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de FLUENZ SUSPENSION PARA PULVERIZACION NASAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes
















