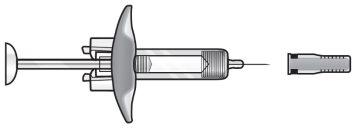
FASENRA 30 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA

Cómo usar FASENRA 30 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Fasenra 30mg solución inyectable en jeringa precargada
benralizumab
Lea todo el prospecto detenidamente antes de empezar a recibir este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Fasenra y para qué se utiliza
- Qué necesita saber antes de empezar a usar Fasenra
- Cómo se administra Fasenra
- Posibles efectos adversos
- Conservación de Fasenra
- Contenido del envase e información adicional
1. Qué es Fasenra y para qué se utiliza
Quées Fasenra
Fasenra contiene el principio activo benralizumab,que es un anticuerpo monoclonal, es decir, un tipo de proteína que reconoce y se une a una sustancia específica en el organismo. La diana de benralizumab es una proteína llamada receptor de interleucina‑5, que se encuentra particularmente en un tipo de glóbulo blanco llamado eosinófilo.
Para qué se utiliza Fasenra
Asma
Fasenra se utiliza para tratar el asma grave eosinofílicaen adultos. El asma eosinofílica es un tipo de asma en el que los pacientes tienen demasiados eosinófilos en sangre o pulmones.
Fasenra se usa junto con otros medicamentos para tratar el asma (altas dosis de «inhaladores de corticosteroides» más otros medicamentos antiasmáticos) cuando la enfermedad no está bien controlada por esos otros medicamentos solos.
Granulomatosis eosinofílica con poliangeítis (GEPA)
Fasenra se utiliza para tratar la GEPA en adultos. La GEPA es una enfermedad en la que las personas tienen demasiados eosinófilos en la sangre y los tejidos y además tienen alguna forma de vasculitis. Esto significa que hay inflamación de los vasos sanguíneos. Esta enfermedad afecta con mayor frecuencia a los pulmones y los senos paranasales, pero a menudo afecta a otros órganos como la piel, el corazón y los riñones.
Cómo actúa Fasenra
Los eosinófilos son glóbulos blancos involucrados en la inflamación del asma y la GEPA. Al unirse a los eosinófilos, Fasenra ayuda a reducir su frecuencia y la inflamación.
Beneficios del uso de Fasenra
Asma
Fasenra puede disminuir la frecuencia de las crisis asmáticas que está experimentando, ayudándole a respirar mejor y disminuyendo sus síntomas del asma. Si está utilizando unos medicamentos denominados «corticosteroides orales», el uso de Fasenra puede permitirle también disminuir la dosis diaria o suspender el tratamiento con los corticosteroides orales que necesite para controlar el asma.
GEPA
Fasenra puede reducir los síntomas y prevenir los brotes de GEPA. Este medicamento también puede permitirle reducir la dosis diaria de corticosteroides orales que necesita para controlar los síntomas.
2. Qué necesita saber antes de empezar a usar Fasenra
No debe usar Fasenra:
- Si es alérgicoa benralizumab o a alguno de los demás componentes de este medicamento (incluidos en la sección 6). Hable con su médico, enfermero o farmacéuticosi piensa que esto le afecta a usted.
Advertencias y precauciones
Consulte a su médico, enfermero o farmacéutico antes de usar Fasenra:
- si tiene una infección parasitariao vive en una zona donde son frecuentes las infecciones por parásitos o viaja a una región de este tipo. Este medicamento puede disminuir su capacidad para combatir determinados tipos de infecciones parasitarias.
- si ha tenido una reacción alérgica a una inyección o medicamento en el pasado(ver sección 4 para consultar los síntomas de una reacción alérgica).
Asimismo, consulte a su médico, farmacéutico o enfermero si está recibiendo Fasenra:
- si su asma permanece descontrolada o empeoradurante el tratamiento con este medicamento.
- si tiene algún síntoma de una reacción alérgica(ver sección 4). Se han producido reacciones alérgicas en pacientes que reciben este medicamento.
Fasenra no es un medicamento de rescate.No lo use para tratar una crisis asmática repentina.
Esté atento a los signos de reacciones alérgicas graves
Fasenra puede potencialmente causar reacciones alérgicas graves. Debe estar atento a los signos de estas reacciones (tales como habones, erupción cutánea, dificultad para respirar, desmayo, malestar, sentirse mareado y/o hinchazón de la cara, lengua o boca) mientras está recibiendo Fasenra.
Es importante que consulte con su médico sobre cómo reconocer los síntomas tempranos de las reacciones alérgicas graves y cómo manejarlas si ocurren.
Con objeto de mejorar la trazabilidad de los medicamentos biológicos, anote el nombre y el número de lote incluidos en el envase exterior y en la etiqueta de la jeringa precargada cada vez que reciba un nuevo envase de Fasenra y proporcione esta información cuando notifique cualquier efecto adverso.
Otros medicamentos para el asma o la GEPA
No suspenda bruscamenteni cambie la dosis de sus otros medicamentos para su enfermedad cuando inicie Fasenra.
Si su respuesta al tratamiento lo permite, su médico puede tratar de reducir la dosis de algunos de estos medicamentos, especialmente los llamados "corticosteroides". Esto debe hacerse gradualmente y bajo la supervisión directa de su médico
Informe a su médicosi está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento antes de usar Fasenra.
Niños y adolescentes
No administre este medicamento a niños menores de 18 años de edad porque se desconocen la seguridad y los beneficios de este medicamento en esta población.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médicoantes de utilizar este medicamento.
No use Fasenra si está embarazada a menos que su médico le diga otra cosa. Se desconoce si Fasenra puede afectar al feto.
No se sabe si los componentes de Fasenra pueden pasar a la leche materna. Si está usted dando el pecho o tiene previsto hacerlo, hable con su médico.
Conducción y uso de máquinas
Es poco probable que Fasenra afecte a su capacidad para conducir y usar máquinas.
Fasenra contiene polisorbato 20
Este medicamento contiene 0,06 mg de polisorbato 20 (de origen vegetal) en cada jeringa precargada de 30 mg. Los polisorbatos pueden causar reacciones alérgicas. Informe a su médico si tiene cualquier alergia conocida.
3. Cómo usar Fasenra
Use siempre este medicamento exactamente como le haya indicado su médico. Consulte con su médico, enfermero o farmacéutico si no está seguro.
Asma
La dosis recomendadaes una inyección de 30 mg. Las 3 primeras inyecciones se administran cada 4 semanas. Con posterioridad, las inyecciones son de dosis de 30 mg cada 8 semanas.
GEPA
La dosis recomendadaes una inyección de 30 mg cada 4 semanas.
Fasenra se administra mediante una inyección justo debajo de la piel (subcutánea). Usted y su médico o enfermero deben decidir si debe inyectarse Fasenra usted mismo. No debe inyectarse Fasenra usted mismo si no ha recibido Fasenra previamente, o si ha tenido una reacción alérgica previa con Fasenra.
Usted o su cuidador deben recibir formación sobre la forma correcta de inyectar Fasenra. Lea cuidadosamente las ‘Instrucciones de Uso’ para la jeringa precargada antes de usar Fasenra.
Siolvidóusar Fasenra
Si ha olvidado inyectarse una dosis de Fasenra, hable con su médico, farmacéutico o enfermero tan pronto como le sea posible.
Siinterrumpeel tratamiento con Fasenra
No interrumpa el tratamiento con Fasenra a menos que su médico se lo recomiende. La interrupción o suspensión del tratamiento con Fasenra puede provocar la reaparición de sus síntomas y exacerbaciones del asma.
Si sus síntomas asmáticos empeoran mientras recibe las inyecciones de Fasenra, hable con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Reaccionesalérgicas graves
Acuda al médico inmediatamentesi cree que puede estar teniendo una reacción alérgica. Dichas reacciones pueden suceder al cabo de horas o días después de la inyección.
Frecuencia no conocida(no puede estimarse a partir de los datos disponibles):
- anafilaxia
Los síntomas habituales incluyen:
o hinchazón en la cara, lengua o boca
o problemas de respiración
o desmayo, mareo, aturdimiento (debido a una bajada en la presión sanguínea)
Frecuentes(pueden afectar hasta 1 de cada 10 personas)
- reacciones de hipersensibilidad (ronchas, erupción cutánea)
Otros efectos adversos
Frecuentes(pueden afectar hasta1 de cada 10personas)
- dolor de cabeza
- faringitis (dolor de garganta)
- fiebre (temperatura elevada)
- reacción en el lugar de inyección (por ejemplo, dolor, enrojecimiento, picor, hinchazón cerca del punto de inyección)
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Fasenra
Mantener este medicamento fuera de la vista y del alcance de los niños.
Fasenra es para un solo uso.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y en la caja después de ‘EXP/CAD’. La fecha de caducidad es el último día del mes que se indica.
Conservar en el envase original para protegerlo de la luz.
Conservar en nevera (entre 2 °C y 8 °C).
La jeringa se puede conservar a temperatura ambiente hasta 25 °C durante un máximo de 14 días. Tras retirarla de la nevera, Fasenra se debe usar en 14 días o desecharse, y se debe escribir la fecha de eliminación en la caja.
No agitar, congelar ni exponer al calor.
Los medicamentos no se deben tirar por los desagües. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composiciónde Fasenra
El principio activo es benralizumab. Una jeringa precargada de 1 ml de solución contiene 30 mg de benralizumab.
Los demás componentes son histidina, hidrocloruro de histidina monohidrato, trehalosa dihidrato, polisorbato 20 (E 432) y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Fasenra es una solución en una jeringa de vidrio transparente. Su color varía entre incoloro y amarillo. Puede contener partículas.
Fasenra está disponible en un envase que contiene 1 jeringa precargada.
Titular de la autorización de comercialización
AstraZeneca AB
SE‑151 85 Södertälje
Suecia
Responsable de la fabricación
AstraZeneca AB
Gärtunavägen
SE-152 57 Södertälje
Suecia
MedImmune UK Ltd
6 Renaissance Way
Liverpool, L24 9JW
Reino Unido
AstraZeneca Nijmegen B.V., Nijmegen
Lagelandseweg 78
Nijmegen, 6545CG
Países Bajos
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien AstraZeneca S.A./N.V. Tel: +32 2 370 48 11 | Lietuva UAB AstraZeneca Lietuva Tel: +370 5 2660550 |
| Luxembourg/Luxemburg AstraZeneca S.A./N.V. Tél/Tel: +32 2 370 48 11 |
Ceská republika AstraZeneca Czech Republic s.r.o. Tel: +420 222 807 111 | Magyarország AstraZeneca Kft. Tel.: +36 1 883 6500 |
Danmark AstraZeneca A/S Tlf.: +45 43 66 64 62 | Malta Associated Drug Co. Ltd Tel: +356 2277 8000 |
Deutschland AstraZeneca GmbH Tel: +49 40 809034100 | Nederland AstraZeneca BV Tel: +31 85 808 9900 |
Eesti AstraZeneca Tel: +372 6549 600 | Norge AstraZeneca AS Tlf: +47 21 00 64 00 |
Ελλ?δα AstraZeneca A.E. Τηλ: +30 210 6871500 | Österreich AstraZeneca Österreich GmbH Tel: +43 1 711 31 0 |
España AstraZeneca Farmacéutica Spain, S.A. Tel: +34 91 301 91 00 | Polska AstraZeneca Pharma Poland Sp. z o.o. Tel.: +48 22 245 73 00 |
France AstraZeneca Tél: +33 1 41 29 40 00 | Portugal AstraZeneca Produtos Farmacêuticos, Lda. Tel: +351 21 434 61 00 |
Hrvatska AstraZeneca d.o.o. Tel: +385 1 4628 000 | România AstraZeneca Pharma SRL Tel: +40 21 317 60 41 |
Ireland AstraZeneca Pharmaceuticals (Ireland) DAC Tel: +353 1609 7100 | Slovenija AstraZeneca UK Limited Tel: +386 1 51 35 600 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika AstraZeneca AB, o.z. Tel: +421 2 5737 7777 |
Italia AstraZeneca S.p.A. Tel: +39 02 00704500 | Suomi/Finland AstraZeneca Oy Puh/Tel: +358 10 23 010 |
Κ?προς Αλ?κτωρ Φαρµακευτικ? Λτδ Τηλ: +357 22490305 | Sverige AstraZeneca AB Tel: +46 8 553 26 000 |
Latvija SIA AstraZeneca Latvija Tel: +371 67377100 | United Kingdom (Northern Ireland) AstraZeneca UK Ltd Tel: +44 1582 836 836 |
Fecha de la última revisión de este prospecto:
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu.
<---------------------------------------------------------------------------------------------------------------->
Instrucciones de uso
Fasenra 30 mg solución inyectable en jeringa precargada
benralizumab
Para inyección subcutánea
Jeringa precargada de un solo uso
Antes de empezar a usar Fasenra jeringa precargada, un profesional sanitario deberá enseñarle a usted o a su cuidador cómo usarla correctamente.
Lea estas “Instrucciones de Uso” antes de empezar a usar Fasenra jeringa precargada y cada vez que tenga que realizar una nueva inyección.Podría haber nueva información. Esta información no sustituye a la consulta con su profesional sanitario respecto a su enfermedad o a su tratamiento.
Si usted o su cuidador tiene cualquier duda, consulte con su profesional sanitario.
Información importante
Conserve Fasenra en una nevera entre 2°C y 8°C en su cartonaje hasta que esté listo para usarlo.Fasenra puede mantenerse a temperatura ambiente hasta 25 °C durante un máximo de 14 días. Tras retirarlo de la nevera, Fasenra se debe usar en 14 días o desecharse.
No usesu jeringa precargada Fasenra si:
| No:
|
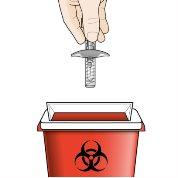
Si ocurre algo de lo anterior, deseche la jeringa en un contenedor para objetos punzantes resistente a las perforaciones y use una nueva jeringa precargada.
Cada jeringa precargada de Fasenra contiene 1 dosis de Fasenra que es de un solo uso.
Mantenga Fasenra y todos los medicamentos fuera de la vista y el alcance de los niños.
Su jeringa precargada Fasenra
No quiteel capuchón de la aguja hasta que haya llegado al Paso 6 y esté listo para inyectar Fasenra.
No toquelos clips de activación del protector de la aguja para evitar activar el dispositivo de seguridad (protector de la aguja) demasiado pronto.
Clips de activación del protector de la aguja | Cuerpo de la jeringa | Etiqueta con la fecha de caducidad | Capuchón de la aguja | |||
Cabeza del émbolo |
| |||||
Émbolo | Reborde para los dedos | Ventana de visión | Aguja |
Paso 1 – Reúna los materiales
- 1 jeringa precargada Fasenra de la nevera
- 1 toallita con alcohol
- 1 bola de algodón o gasa
- 1 contenedor para objetos punzantes.
(Vea el paso 9 – Desechar la jeringa precargada)
|
|
|
|
Jeringa precargada | Toallita con alcohol | Bola de algodón o gasa | Contenedor para objetos punzantes |
Paso2 – Prepárese para usar su jeringa precargada | |
Compruebe la fecha de caducidad (CAD).No la use si la fecha de caducidad ha pasado. Antes de la administración, deje que la jeringa precargada alcance una temperatura ambiente de 20°C a 25°C dejando el cartonaje fuera de la nevera durante aproximadamente 30minutos. Nocaliente la jeringa precargada de ninguna otra manera. Por ejemplo, no la caliente en un microondas o con agua caliente ni lo ponga cerca de fuentes de calor. Use Fasenra en los 14 días tras sacarlo de la nevera. |
|
Paso 3 – Compruebe el líquido | |
Agarre el cuerpo de la jeringa(noel émbolo) para retirar la jeringa. Observe el líquido a través de la ventana de visión.El líquido debe ser claro y de incoloro a amarillento. Puede que contenga pequeñas partículas blancas. Noinyecte Fasenra si el líquido está turbio, decolorado o si contiene partículas de gran tamaño. Puede que vea una pequeña burbuja de aire en el líquido. Esto es normal. No necesita hacer nada al respecto. |
|
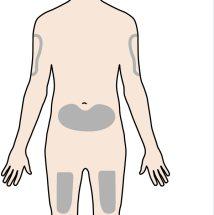
Paso 4 –Escoja el lugar de la inyección | |
El lugar recomendado para la inyección es la parte anterior del muslo. Usted puede usar también la parte baja del abdomen. Nolo inyecte:
Un cuidador puede inyectárselo en la parte superior del brazo, muslo o abdomen. Notrate de inyectárselo usted mismo en la parte superior del brazo. Para cada inyección elija un lugar diferente, separados al menos 3 cm del lugar donde se realizó la inyección anterior. |
|
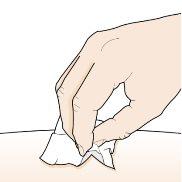
Paso 5 – Limpie el lugar de la inyección | |
Lávese bien las manos con agua y jabón. Limpie el lugar de la inyección con una toallita con alcohol con un movimiento circular. Deje que se seque al aire. Notoque el área limpiada antes de la inyección. Noabanique ni sople el área limpiada. |
|
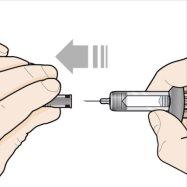
Paso 6 – Retire el capuchón de la aguja | |
Sujete la jeringa con una mano, y tire con cuidado del capuchón de la aguja con la otra mano. Nosujete el émbolo ni la cabeza del émbolo mientras retira el capuchón de la aguja. Deje el capuchón de la aguja a un lado para tirarlo más tarde. Puede que vea una gota de líquido al final de la aguja. Esto es normal. Nouse la jeringa si se ha caído sin el capuchón de la aguja en su sitio o si la aguja está dañada o sucia. Notoque la aguja, ni deje que toque ninguna superficie. Siga directamente con los próximos pasos, sin pausa. |
|
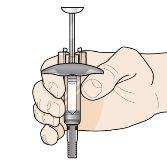
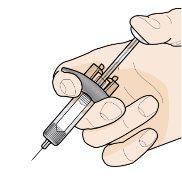
Paso 7 – Inyecte Fasenra | |
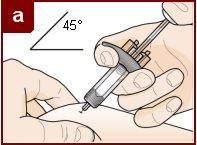
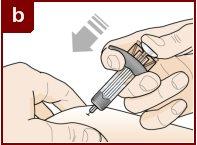
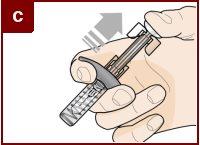
Sujete la jeringa precargada con una mano, como se muestra en la figura. Use la otra mano para pellizcar y sujetar suavemente el área de la piel donde quiere inyectar. Esto crea una superficie más firme. Nopresione el émbolo hasta que la aguja esté completamente insertada en la piel. Notire hacia atrás del émbolo en ningún momento. Inyecte Fasenra siguiendo los pasos de las figuras a, by c. |
|
|
|
|
Realice un movimiento rápido, como un dardo, para insertar la aguja en la piel pellizcada. Inserte la aguja con un ángulo de 45 grados. | Use su pulgar para empujar la cabeza del émbolo. Siga empujando el émbolo hasta el fondo. Esto es para asegurarse de que se ha inyectado toda la medicación. | Mantenga su pulgar empujando la cabeza del émbolo mientras retira la aguja de la piel. Deje de ejercer presión sobre el émbolo ligeramente hasta que los clips de activación del protector de la aguja cubran a ésta. |
Paso 8 – Compruebe el lugar de la inyección | |
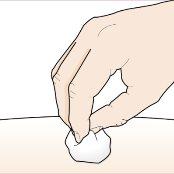
Puede que haya una pequeña cantidad de sangre o líquido donde se ha inyectado. Esto es normal. Presione suavemente sobre la piel con una bola de algodón o una gasa hasta que pare el sangrado. Nofrote el lugar de la inyección. Si fuera necesario, cubra el lugar de la inyección con un pequeño apósito. |
|
Paso 9 – Deseche la jeringa precargada usada | |
Notire la jeringa precargada en su basura doméstica. Novuelva a tapar la jeringa precargada. Tire el capuchón de la aguja y cualquier otro material usado en su basura doméstica. |
|
Guía para desechos Deshágase del contendedor en su conjunto acorde a las instrucciones de su profesional sanitario o farmacéutico. Norecicle su contenedor de objetos punzantes. |
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a FASENRA 30 MG SOLUCION INYECTABLE EN JERINGA PRECARGADAForma farmacéutica: INYECTABLE, 30 mgPrincipio activo: BenralizumabFabricante: Astrazeneca AbRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, 100 mg inyectable 10 mlPrincipio activo: ReslizumabFabricante: Teva B.V.Requiere recetaForma farmacéutica: COMPRIMIDO, 250 µgPrincipio activo: roflumilastFabricante: Astrazeneca AbRequiere receta
Médicos online para FASENRA 30 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de FASENRA 30 MG SOLUCION INYECTABLE EN JERINGA PRECARGADA, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes