
DACEPTON 10 MG/ML SOLUCION INYECTABLE EN CARTUCHO EFG

Cómo usar DACEPTON 10 MG/ML SOLUCION INYECTABLE EN CARTUCHO EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
- Introducción
- Qué es Dacepton 10 mg/ml en cartucho y para qué se utiliza
- Qué necesita saber antes de empezar a usar Dacepton 10 mg/ml en cartucho
- Cómo usar Dacepton 10 mg/ml en cartucho
- Posibles efectos adversos
- Conservación de Dacepton 10 mg/ml en cartucho
- Contenido del envase e información adicional
- Composición de Dacepton 5 mg/ml
Introducción
Prospecto: Información para el usuario
Dacepton 10 mg/ml solución inyectable en cartucho EFG
Apomorfina, hidrocloruro hemihidrato
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
El nombre de su medicamento es Dacepton 10 mg/ml solución inyectable en cartucho EFG, y se le denominará Dacepton 10 mg/ml en cartucho a lo largo de todo este prospecto.
Contenido del prospecto
- Qué es Dacepton 10 mg/ml en cartucho y para qué se utiliza
- Qué necesita saber antes de empezar a usar Dacepton 10 mg/ml en cartucho
- Cómo usar Dacepton 10 mg/ml en cartucho
- Posibles efectos adversos
- Conservación de Dacepton 10 mg/ml en cartucho
- Contenido del envase e información adicional
1. Qué es Dacepton 10 mg/ml en cartucho y para qué se utiliza
Dacepton contiene una solución inyectable de apomorfina. Se inyecta en el área bajo la piel (subcutáneamente) utilizando solamente la pluma D-mine Pen destinada para ello. El principio activo de Dacepton es apomorfina hidrocloruro hemihidrato. Cada mililitro de solución contiene 10 mg de apomorfina hidrocloruro hemihidrato.
La apomorfina hidrocloruro hemihidrato pertenece a un grupo de medicamentos conocidos como agonistas de la dopamina. Dacepton se utiliza para tratar la enfermedad de Parkinson. La apomorfina ayuda a reducir el tiempo en estado ‘off’ o de inmovilidad de las personas tratadas previamente para la enfermedad de Parkinson con levodopa (otro tratamiento para la enfermedad de Parkinson) y/o con otros agonistas de la dopamina.
Su médico o su enfermera le ayudarán a reconocer los signos que le indicarán cuándo debe utilizar su medicamento.
A pesar de su nombre, la apomorfina no contiene morfina.
2. Qué necesita saber antes de empezar a usar Dacepton 10 mg/ml en cartucho
NO use Dacepton 10 mg/ml en cartucho:
- si es alérgico a la apomorfina o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- si es menor de 18 años.
- si tiene dificultades para respirar.
- si sufre demencia o enfermedad de Alzheimer.
- si tiene alguna enfermedad mental con síntomas como alucinaciones, delirios, pensamientos confusos, pérdida de contacto con la realidad.
- si tiene problemas de hígado.
- si sufre discinesia grave (movimientos involuntarios) o distonía grave (incapacidad para moverse) a pesar de estar tomando levodopa.
- si sabe que usted o alguien de su familia presentan una alteración de electrocardiograma (ECG) denominada ‘síndrome del intervalo QT largo’. Informe a su médico.
- si está tomando ondansetrón (medicamento para tratar las náuseas y el vómito).
Advertencias y precauciones
Antes de usar Dacepton, su médico le hará un ECG (electrocardiograma), y le pedirá una lista de todos los demás medicamentos que toma. Este ECG se repetirá en los primeros días del tratamiento, y en cualquier momento en que su médico lo considere necesario. También le preguntará sobre otras enfermedades que pueda tener, en especial relacionadas con el corazón. Puede que algunas de las preguntas y exploraciones complementarias se repitan en cada visita médica. Si tiene síntomas que pueden proceder del corazón, por ejemplo palpitaciones, desmayo o mareos, debe comunicárselo a su médico de inmediato. Si tiene diarrea o comienza a usar un medicamento nuevo, también debe comunicárselo a su médico.
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar Dacepton:
- si sufre problemas de riñón
- si sufre problemas de pulmón
- si sufre problemas de corazón
- si tiene la presión arterial baja o se siente débil o mareado al ponerse de pie
- si está tomando medicamentos para tratar la tensión arterial elevada
- si tiene náuseas o sensación de estar mareado
- si la enfermedad de Parkinson le causa algún problema mental como alucinaciones y confusión.
- si es de edad avanzada o se encuentra débil
Informe a su médico si usted, su familia o su cuidador observa que presenta impulsos o deseos irrefrenables de comportarse de manera no habitual en usted y que no puede resistir el impulso, el deseo o la tentación de realizar ciertas actividades que podrían dañarle a usted mismo o a otras personas. Estos se denominan trastornos de control de los impulsos y pueden incluir conductas como juego adictivo, comer o gastar dinero en exceso, un deseo sexual anormalmente alto o un aumento de los pensamientos o los sentimientos sexuales. Es posible que el médico deba ajustar o interrumpir la dosis.
Algunos pacientes desarrollan síntomas de adicción que les llevan a un deseo compulsivo de consumir dosis elevadas de Dacepton y otros medicamentos empleados en el tratamiento de la enfermedad de Parkinson.
Niños y adolescentes
Dacepton no se debe utilizar en niños y adolescentes menores de 18 años.
Uso de Dacepton 10 mg/ml en cartucho con otros medicamentos
Comunique a su médico o su farmacéutico si está tomando, ha tomado recientemente o pudiera tener que tomar cualquier otro medicamento.
Consulte a su médico o farmacéutico antes de tomar su medicamento
Si está tomando medicamentos que se sabe que afectan a la forma en que late su corazón. Esto incluye los medicamentos que se usan para tratar problemas del ritmo cardíaco (como la quinidina y la amiodarona), para la depresión (incluidos los antidepresivos tricíclicos, como la amitriptilina y la imipramina) y para infecciones bacterianas (antibióticos ‘macrólidos’ como la eritromicina, la azitromicina y la claritromicina) y la domperidona.
Si está tomando ondansetrón (medicamento para tratar las náuseas y el vómito), ya que esto puede resultar en una disminución grave de la presión arterial y pérdida de la consciencia.
Si usa Dacepton con otros medicamentos, el efecto de esos medicamentos puede verse alterado.
Esto sucede especialmente con:
- Medicamentos como la clozapina para tratar algunos trastornos mentales.
- Medicamentos para reducir la tensión arterial.
- Otros medicamentos para la enfermedad de Parkinson.
Su médico le indicará si debe ajustar la dosis de apomorfina o de cualquier otro medicamento que esté utilizando.
Si está tomando levodopa (otro medicamento para la enfermedad de Parkinson) además de apomorfina, su médico debería realizarle un análisis de sangre periódicamente.
Uso de Dacepton 10 mg/ml en cartucho con alimentos y bebidas
Los alimentos y las bebidas no afectan la forma en la que Dacepton actúa.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Dacepton no debe usarse durante el embarazo a menos que sea estrictamente necesario.
No se sabe si el Dacepton se transfiere a la leche materna. Consulte a su médico si está en periodo de lactancia o si piensa dar el pecho. El médico le explicará si debe continuar o interrumpir la lactancia o si debe continuar o interrumpir este medicamento.
Conducción y uso de máquinas
Dacepton puede causar somnolencia y un fuerte deseo de dormir. No conduzca ni maneje herramientas ni máquinas si Dacepton le produce este efecto.
Dacepton 10 mg/ml en cartucho contiene metabisulfito de sodio
El metabisulfito de sodio en raras ocasiones puede producir reacciones alérgicas graves con síntomas tales como sarpullido o picor en la piel, dificultad para respirar, hinchazón de los párpados, la cara o los labios, inflamación o enrojecimiento de la lengua. Si sufre estos efectos adversos, acuda inmediatamente al servicio de urgencias del hospital más cercano.
Este medicamento contiene menos de 23 mg (1 mmol) de sodio por 10 ml; esto es, esencialmente “exento de sodio”.
3. Cómo usar Dacepton 10 mg/ml en cartucho
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Antes de usar Dacepton su médico verificará que tolera este medicamento y un medicamento antiemético que debe tomar simultáneamente.
Debe tomar domperidona durante al menos 2 días antes de comenzar con Dacepton 10 mg/ml para evitar tener náuseas o sentirse mareado.
NO use Dacepton 10 mg/ml en cartucho si
- la solución se ha puesto de color verde.
- la solución está turbia o se pueden ver partículas en ella.
Cuánta cantidad debe usarse
Tanto la cantidad de Dacepton que debe usar como el número de inyecciones diarias necesarias dependerán de sus necesidades personales. Su médico lo analizará con usted y le dirá la cantidad de medicamento que debe administrarse y con qué frecuencia.
La cantidad más adecuada para usted se determinará durante su visita al especialista.
- La dosis diaria habitual varía entre 3 mg y 30 mg.
- Puede que necesite hasta 100 mg al día.
- Normalmente necesitará entre 1 y 10 inyecciones al día.
- Cada inyección individual no debe contener más de 10 mg.
La pluma D-minePen que se necesita para la aplicación de Dacepton 10 mg/ml en cartucho, no es adecuada para pacientes que necesitan dosis superiores a 6 mg por inyección.
Para estos pacientes se deben utilizar otros productos.
No es necesario diluir Dacepton antes de su uso. Además, no debe mezclarse con ningún otro medicamento.
- El médico le indicará la dosis de Dacepton que debe usar y con qué frecuencia. También le informará de cómo cambiar la dosis de Dacepton, si fuera necesario. No cambie su dosis de Dacepton ni la use con mayor frecuencia, a no ser que se lo indique su médico.
- El médico le proporcionará a usted y a sus cuidadores las instrucciones detalladas sobre la preparación y la administración de las dosis, prestando una atención especial al uso correcto de la pluma de administración requerida.
Antes de usar Dacepton 10 mg/ml en cartucho
Nota: este envase NO incluye la pluma ni las agujas de la pluma.
Dacepton 10 mg/ml en cartucho está diseñado para ser administrado solamente con la pluma “D-minePen” destinada para ello y agujas desechables, como se especifica en las Instrucciones de uso de la pluma.
Descripción de la pluma
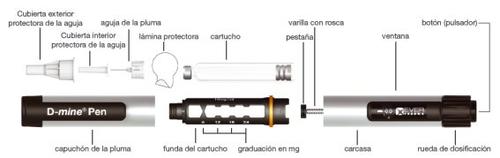
- Use siempre una aguja nueva para cada inyección, para prevenir la contaminación.
- No deben compartirse las agujas ni la pluma.
Antes de usar Dacepton 10 mg/ml en cartucho, examine su pluma y el manual de la pluma para familiarizarse con su correcta manipulación.
- Si su pluma está dañada o no funciona correctamente (debido a defectos mecánicos), lea las Instrucciones de uso de la pluma.
Dónde y cómo inyectar Dacepton 10 mg/ml en cartucho
- Primero lávese las manos.
- Antes de usar la pluma, necesitará toallitas quirúrgicas y una aguja con su cono protector.
- Siga las instrucciones de su manual de la pluma.
Preparación de la pluma / cambio del cartucho
Saque la pluma de su envase y quite el capuchón.

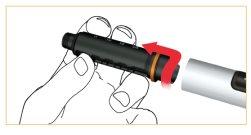
Retire la funda del cartucho girando en dirección a las agujas del reloj
Inserte el nuevo cartucho en la funda
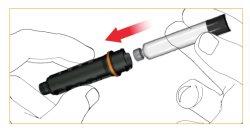
Empuje hasta el fondo la varilla con rosca. Esto se realiza mejor con la punta del dedo.
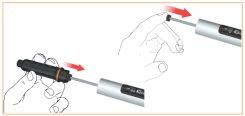
Meta la funda del cartucho en la carcasa y gire en dirección contraria a las agujas del reloj para encajarla

Ajuste de la aguja del cartucho
Siga las instrucciones de uso de la aguja de la pluma. Retire la lámina protectora.
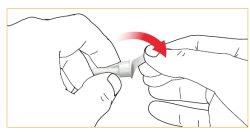
Encaje /gire la aguja de la pluma a la cubierta del cartucho.
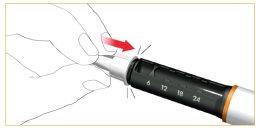
Retire la cubierta exterior protectora de la aguja. Guárdela para eliminar la aguja del cartucho de forma segura después de su uso.
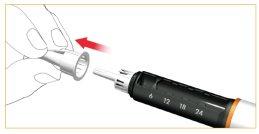
Retire y deseche la cubierta interior protectora de la aguja

Control del cebado / funcionamiento
Todo el aire del cartucho debe eliminarse antes de usarlo. Marque la dosis hacia delante girando la rueda de dosificación. Controle la dosis marcada mirando la ventana en vertical desde arriba, y no en ángulo, de manera que el símbolo “?“ se vea claramente. Esto se denomina “cebado” y es importante para asegurar que usted recibe una dosis completa cuando utiliza la pluma

Para controlar el funcionamiento, sujete la pluma apuntando hacia arriba y golpee suavemente la funda del cartucho, de manera que el aire suba hacia arriba.
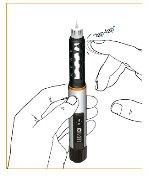
Empuje el botón

Saldrán algunas gotitas por la punta de la aguja de la pluma. Si no salen gotitas, repita este paso.

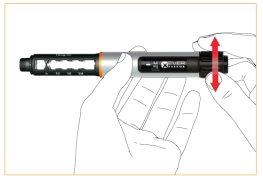
Ajuste de la dosis
Marque su dosis haciendo girar la rueda de dosificación en dirección a las agujas del reloj. Corrija su dosis girando en dirección contraria a las agujas del reloj.
Inyección
- Con la ayuda de una toallita quirúrgica, limpie el área de la piel donde piensa inyectar el medicamento y alrededores.
- Inyecte Dacepton en el área central de su cintura (abdomen) o en la parte exterior del muslo, debajo de la piel (subcutáneamente) como le indicó su médico o enfermera.
Para la inyección, empuje el botón hasta el final. Mantenga el botón presionado durante la descarga del medicamento. Una vez que se ha descargado completamente su medicación, espere 6 segundos y entonces retire lentamente la aguja de la pluma. Usted puede mantener el botón presionado o liberarlo durante los 6 segundos. Compruebe en la ventana que se ve la posición “0,0”, para confirmar que se ha puesto la dosis completa. 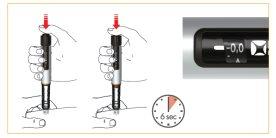
- Cambie el lugar de la inyección cada vez que use Dacepton 10 mg/ml en cartucho. Esto disminuirá las probabilidades de que aparezca una reacción cutánea en el lugar donde inyectó Dacepton. No inyecte Dacepton en un área de la piel que esté dolorida, roja, infectada o dañada.
- Nunca debe inyectarse directamente en una vena (intravenosamente) o músculo (intramuscularmente).
Después de usar Dacepton 10 mg/ml en cartucho
Elimine y deseche la aguja después de cada inyección (para ver como eliminarla de manera segura ver sección 5).
Eliminación de la aguja de la pluma después de cada inyección
Coloque con cuidado la cubierta exterior protectora sobre la aguja de la pluma.
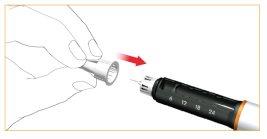
Desenrosque la aguja de la pluma girando la carcasa exterior en sentido de las agujas del reloj y elimínela correctamente.

Opcional:
Coloque la cubierta exterior de la aguja de la pluma en el hueco correspondiente de su estuche. La apertura de la cubierta de la aguja debe apuntar hacia arriba. Inserte con cuidado la aguja (unida a la pluma) en la apertura del estuche. Sin tocar la cubierta, empuje hacia abajo firmemente y gire en sentido contrario a las agujas del reloj para desenroscar la aguja de la pluma.
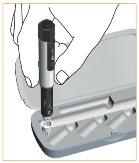
Cierre bien con el capuchón de la pluma después de cada uso.
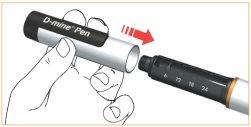
- Deje el cartucho en su pluma.
- Se puede usar un nuevo cartucho durante un máximo de 15 días (para más información ver sección 5 “Conservación de Dacepton 10 mg/ml en cartucho”).
- Si no queda suficiente solución para su siguiente dosis, elimine y deseche el cartucho
- Deseche la aguja de una forma segura, como se describe en las Instrucciones de uso de su pluma.
Si usa más Dacepton 10 mg/ml en cartucho del que debe
- Informe a su médico o contacte con el servicio de urgencias del hospital más cercano inmediatamente.
- Puede notar que le late el corazón más despacio, un mareo excesivo, exceso de somnolencia y/o dificultad para respirar. También es posible que se sienta débil o mareado especialmente al ponerse de pie, debido a una bajada de la tensión arterial. Tumbarse con los pies elevados puede ayudarle a combatir la tensión arterial baja.
En caso de sobredosis o ingestión accidental consulte inmediatamente a su médico o llame al Servicio de Información toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó usar Dacepton 10 mg/ml en cartucho
Adminístrese la siguiente dosis cuando lo necesite. No use una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Dacepton 10 mg/ml en cartucho
No interrumpa el tratamiento con Dacepton sin consultar previamente con su médico.
Si tiene alguna cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Si experimenta una reacción alérgica, interrumpael uso de Dacepton 10 mg/ml en cartucho y contacte con su médico o su servicio de urgencias del hospital más cercano inmediatamente.
Los signos de una reacción alérgica pueden incluir:
- Sarpullido
- Dificultad para respirar u
- Opresión en el pecho,
- Hinchazón de los párpados, cara o labios,
- Inflamación o enrojecimiento de la garganta o la lengua.
Dacepton puede a veces producir los siguientes efectos adversos.
Efectos adversos muy frecuentes (pueden afectar a más de 1 de cada 10 pacientes):
- Bultos bajo la piel en el lugar de la inyección que duelen, molestan y pueden ponerse rojos y picar. Para evitar tener estos bultos, es aconsejable cambiar el lugar de inyección cada vez que se inserte la aguja.
- Alucinaciones (ver, oír o sentir cosas inexistentes)
Efectos adversos frecuentes (pueden afectar hasta 1 de cada 10 pacientes):
- Náuseas o vómitos, especialmente al comienzo. Si está tomando domperidona y aún siente náuseas, o si no está tomando domperidona y se siente mareado, informe a su médico o a su enfermera lo antes posible.
- Cansancio o somnolencia excesiva.
- Confusión o alucinaciones.
- Bostezos.
- Sensación de mareo o de ligero mareo al ponerse en pie
Efectos adversos poco frecuentes (pueden afectar hasta 1 de cada 100 pacientes):
- Aumento de los movimientos involuntarios o aumento de los temblores durante los períodos de ‘on’.
- Anemia hemolítica, una destrucción anormal de los glóbulos rojos en los vasos sanguíneos o en otras partes del cuerpo. Este es un efecto adverso poco frecuente que puede producirse en pacientes que también toman levodopa.
- Quedarse dormido súbitamente.
- Sarpullido.
- Dificultad para respirar.
- Úlceras en el lugar de la inyección.
- Reducción de los glóbulos rojos que puede hacer que la piel esté amarillenta y causar debilidad o falta de respiración.
- Reducción de las plaquetas de la sangre, que aumenta el riesgo de sangrado o hematomas.
Efectos adversos raros (pueden afectar hasta 1 de cada 1000 pacientes):
- Una reacción alérgica
- Eosinofilia, una cantidad anormalmente elevada de glóbulos blancos en la sangre o en los tejidos del cuerpo.
Efectos adversos de frecuencia no conocida (no puede estimarse a partir de los datos disponibles):
- Hinchazón de las piernas, los pies o los dedos de las manos.
- Desmayos
- Agresividad, agitación
- Dolor de cabeza
- Incapacidad para resistir el impulso, el deseo o la tentación de realizar una acción que podría ser perjudicial para usted o para otras personas, que puede incluir:
- Fuerte impulso para jugar en exceso, a pesar de las consecuencias graves personales o familiares.
- Alteración o aumento del interés y el comportamiento sexual, significativo para usted o para otras personas, por ejemplo, aumento del impulso sexual.
- Compras o gastos excesivos incontrolables.
- Atracones de comida (comer grandes cantidades de alimentos en corto espacio de tiempo) o comer de forma compulsiva (comer más comida de lo normal y más de lo necesario para satisfacer el hambre).
Informe a su médico si se presenta alguna de estas conductas; él o ella le indicarán la forma de controlar o reducir los síntomas.
Comunicación de efectos adversos:
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Dacepton 10 mg/ml en cartucho
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja y en la etiqueta después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
No conservar a temperatura superior a 25ºC.
No refrigerar o congelar.
Mantenga los cartuchos en el embalaje exterior para protegerlos de la luz.
Conservar en las mismas condiciones una vez abierto y entre aperturas.
Cuando comience a usar un nuevo cartucho, este puede usarse hasta durante 15 días. No reutilice el cartucho después de este periodo de tiempo. Use un nuevo cartucho.
No utilice este medicamento si observa que la solución se ha puesto de color verde. Sólo debe utilizarse si la solución es transparente, incolora o ligeramente amarillenta y no contiene partículas.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de su farmacia habitual. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Dacepton 5 mg/ml
El principio activoes apomorfina hidrocloruro hemihidrato. Cada mililitro de Dacepton 5 mg/ml contiene 5 mg de apomorfina hidrocloruro hemihidrato.
Dacepton 5 mg/ml se presenta en viales de 20 ml que contienen 100 mg de apomorfina hidrocloruro hemihidrato.
Los demás componentes (excipientes) son:
- Metabisulfito de sodio (E223)
- Cloruro de sodio
- Ácido clorhídrico (para ajuste del pH)
- Agua para preparaciones inyectables
Consulte en la sección 2: Dacepton 5 mg/ml contiene metabisulfito de sodio (E223) y cloruro de sodio’ con respecto al metabisulfito sódico y al cloruro de sodio.
Aspecto del producto y contenido del envase
Dacepton 5 mg/ml es una solución para perfusión, transparente e incolora a ligeramente amarillenta.
Viales de vidrio con 20 ml de solución para perfusión, en cajas de 1,5 o 30 viales.
Tamaños de envase : 5 x 1, 10 x 1, 30 x 1, 2 x 5 y 6 x 5
Puede que solamente estén comercializados algunos tamaños de envase.
Titular de la autorización de comercialización
EVER Neuro Pharma GmbH
A-4866 Unterach
Austria
Responsable de la fabricación
EVER Pharma Jena GmbH
Brüsseler Strasse 18
07747 Jena
Alemania
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de autorización de comercialización:
EVER Pharma Therapeutics Spain, S.L.
C/Toledo 170
28005 Madrid
España
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeoy en el Reino Unido e Irlanda del Norte, con los siguientes nombres:
AT Dacepton® 5mg/ml Infusionslösung
BE Dacepton® 5 mg /ml oplossing voor infusie
BG Dacepton® 5mg/ml ?????????? ???????
CZ Dacepton® 5mg/ml Infuzní roztok
DE Dacepton® 5mg/ml Infusionslösung
DK Dacepton® 5 mg /ml infusionsvæske, opløsning
EE Dacepton® 5 mg /ml
EL Dopaceptin® 5 mg /ml Δι?λυμαγια?γχυση
ES Dacepton® 5mg/ml Solución para perfusión
FI Dacepton® 5 mg /ml infuusioneste, liuos
FR Dopaceptin® 5 mg /ml Solution pour perfusion
HU Dacepton® 5 mg /ml Oldatos infúzió
IE Dacepton® 5 mg /ml solution for infusion
IT Dacepton®
LT Dacepton® 5mg/ml Infuzinis tirpalas
LV Dacepton® 5mg/ml škidums infuzijam
NL Dacepton® 5 mg /ml oplossing voor infusie
NO Dacepton®
PL Dacepton®
PT Dacepton®
SE Dacepton® 5 mg /ml infusionsvätska, lösning
SI Dacepton® 5 mg /ml raztopina za infundiranje
SK Dacepton® 5mg/ml Infúzny roztok
UK (Northern Ireland) Dacepton® 5 mg /ml solution for infusion
Fecha de la última revisión de este prospecto: Octubre 2023
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS)http://www.aemps.gob.es/
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a DACEPTON 10 MG/ML SOLUCION INYECTABLE EN CARTUCHO EFGForma farmacéutica: INYECTABLE, 10 MG/MLPrincipio activo: apomorphineFabricante: Stada Arzneimittel AgRequiere recetaForma farmacéutica: INYECTABLE, 10 mg/ml apomorfina hidrocloruroPrincipio activo: apomorphineFabricante: Stada Arzneimittel AgRequiere recetaForma farmacéutica: INYECTABLE PERFUSION, 5 MG/MLPrincipio activo: apomorphineFabricante: Stada Arzneimittel AgRequiere receta
Médicos online para DACEPTON 10 MG/ML SOLUCION INYECTABLE EN CARTUCHO EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de DACEPTON 10 MG/ML SOLUCION INYECTABLE EN CARTUCHO EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes








