CLINIMIX N9G15E SOLUCION PARA PERFUSION

Cómo usar CLINIMIX N9G15E SOLUCION PARA PERFUSION
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Clinimix N9G15E solución para perfusión
Lea todo el prospecto detenidamente antes de iniciar la administración de este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o enfermero.
- Si experimenta efectos adversos, consulte a su médico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto:
- Qué es CLINIMIX y para qué se administra
- Qué necesita saber antes de iniciar la administración de CLINIMIX
- Cómo se administra CLINIMIX
- Posibles efectos adversos
- Conservación de CLINIMIX
- Contenido del envase e información adicional
1. Qué es Clinimix y para qué se utiliza
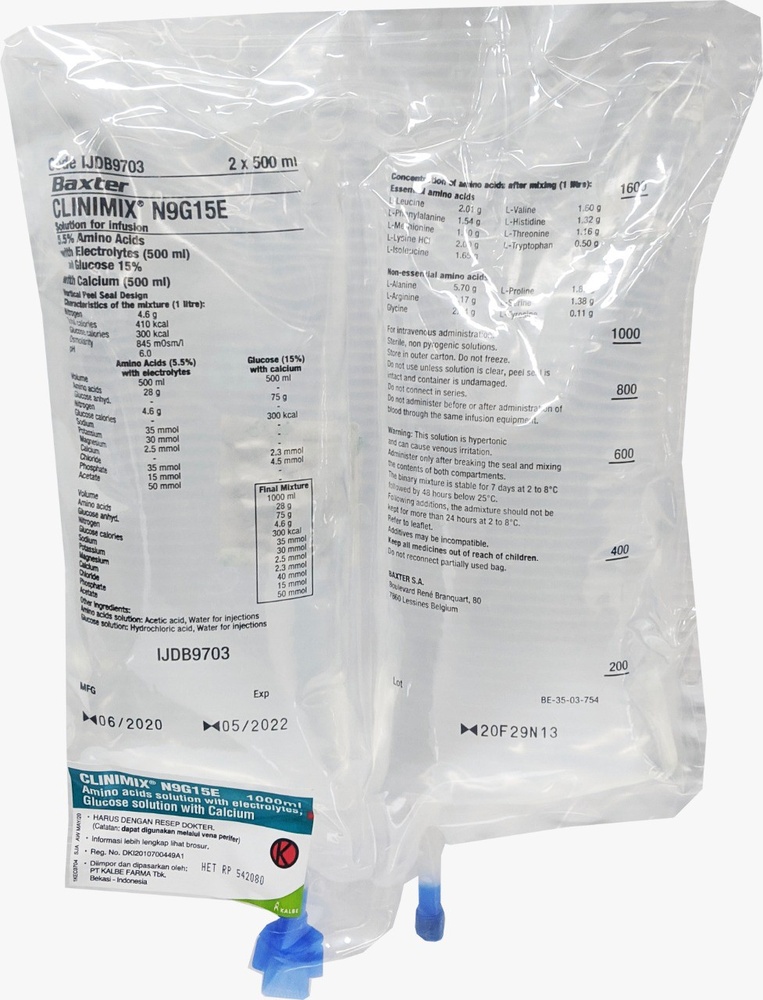
CLINIMIX es una solución para perfusión. Se suministra en una bolsa con 2 cámaras. Una cámara contiene una solución de aminoácidos con electrolitos y la segunda contiene una solución de glucosa con cloruro de calcio. Las cámaras están separadas mediante un sello no permanente. El contenido de las cámaras debe mezclarse inmediatamente antes de la administración enrollando la parte superior de la bolsa para abrir los sellos.
CLINIMIX es administrado para alimentar a adultos y niños mediante un tubo conectado a una vena cuando la alimentación normal por vía oral no es adecuada.
CLINIMIX sólo debe ser administrado bajo supervisión médica.
2. Qué necesita saber antes de iniciar la administración de Clinimix
CLINIMIX no debe ser administrado si:
- es alérgico a los principios activos o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6),
- su organismo tiene problemas para utilizar ciertos aminoácidos,
- usted tiene demasiado azúcar en la sangre (hiperglucemia grave),
- su sangre es excesivamente ácida (acidosis metabólica debido a un exceso de lactato),
- tiene un nivel de sodio, potasio, magnesio, calcio y/o fósforo en sangre demasiado alto (hipernatremia, hiperpotasemia, hipermagnesemia, hipercalcemia y/o hiperfosfatemia),
- en niños de menos de 28 días no se debe co-administrar ceftriaxona con soluciones intravenosas que contengan calcio, porque se pueden formar partículas.
En todos los casos, su médico decidirá si se le debe administrar este medicamento en función de factores como la edad, peso y estado clínico, junto con los resultados de todas las pruebas realizadas.
Advertencias y precauciones
Consulte a su médico o enfermero antes de que le administre CLINIMIX.
Si se desarrolla cualquier signo anormal o hay síntomas de una reacción alérgica, como fiebre, escalofríos, erupciones cutáneas o dificultad para respirar, sudoración excesiva, náuseas y dolor de cabeza, dígaselo a su médico o enfermero: la perfusión se detendrá inmediatamente. Su médico supervisará su estado mientras se le administra este medicamento y puede cambiar la dosis o añadirle otros nutrientes, como lípidos, vitaminas, electrolitos y oligoelementos si lo considera adecuado.
Ciertos medicamentos y enfermedades pueden aumentar el riesgo de desarrollar una infección o sepsis (bacterias en la sangre). Hay un riesgo especial de infección o sepsis, cuando un tubo (catéter intravenoso) se coloca en su vena. Su médico le vigilará cuidadosamente para detectar cualquier signo de infección. El uso de técnicas asépticas (libre de gérmenes) a la hora de colocar y mantener el catéter y al hacer la fórmula nutricional puede reducir el riesgo de infección.
CLINIMIX con electrolitos contiene calcio. No debe administrarse conjuntamente con el antibiótico Ceftriaxona porque se pueden formar partículas.
Si usted está severamente desnutrido de manera tal que necesita recibir alimentación a través de una vena, se recomienda que la nutrición parenteral se inicie lentamente y con cuidado.
Su médico supervisará su estado al inicio de la perfusión, sobre todo si actualmente padece de problemas en el hígado, riñón, adrenales, corazón o en la circulación. Su médico también debe ser consciente de las condiciones graves que afectan a cómo el cuerpo maneja los azúcares, grasas, proteínas o sales (trastornos metabólicos). Si se produce algún signo anormal, entre los que se incluye la irritación venosa, deberá detenerse la perfusión.
Para comprobar la eficacia y la seguridad de la administración, su médico le realizará pruebas de laboratorio y clínicas mientras se le administre este medicamento. Si se le administra este medicamento durante varias semanas, se analizará regularmente su sangre. En particular, en caso de intolerancia a la glucosa, se controlarán de forma rutinaria la glucosa en sangre y en la orina, y, si usted es un paciente diabético, la dosis de insulina puede tener que adaptarse.
Niños y adolescentes
Cuando se utilice en recien nacidos y niños menores de 2 años, la solución (en las bolsas y en los equipos de administración) debe protegerse de la exposición a la luz hasta que se complete la administración. La exposición de Clinimix a la luz ambiental, especialmente después de mezclas con oligoelementos y/o vitaminas, genera peróxidos y otros productos de degradación que pueden reducirse mediante la protección de la exposición a la luz.
Interacción de CLINIMIX con otros medicamentos
Informe a su médico o enfermero si está tomando o ha tomado recientemente o podría tener que tomar cualquier otro medicamento.
CLINIMIX con electrolitos contiene calcio. No debe administrarse junto con el antibiótico ceftriaxona porque se pueden formar partículas.
Debido al contenido de potasio de CLINIMIX, se debe prestar atención especial con pacientes tratados con diuréticos ahorradores de potasio (por ejemplo, amilorida, espironolactona, triamtereno), inhibidores del enzima convertidor de angiotensina (IECA), antagonistas de los receptores de angiotensina II o los inmunosupresores tacrolimus o ciclosporina en vista del riesgo de hiperpotasemia.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, o cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico antes de utilizar este medicamento.
3. Cómo se administra Clinimix
Antes de administrar el producto, se debe romper el sello no permanente entre los dos compartimentos y mezclar el contenido de ambos.
CLINIMIX puede administrarse a adultos y niños.
Se trata de una solución para perfusión que se administra mediante un tubo de plástico acoplado a una vena de su brazo o a una vena grande de su pecho.
Cuando se utilice en recién nacidos y niños menores de 2 años, la solución (en las bolsas y equipos de administración) se debe proteger de la exposición a la luz hasta que finalice la administración (ver sección 2).
Dosis – Adultos y niños
Su médico decidirá la dosis que necesitará y el tiempo durante el que se le administrará, en función de la edad, peso y altura, estado clínico, volumen diario de líquidos y necesidades de energía y nitrógeno.
Siga exactamente las instrucciones de administración de CLINIMIX indicadas por su médico. Consulte a su médico si tiene dudas.
La administración puede continuar durante tanto tiempo como sea necesario, en función de su estado clínico.
La perfusión de una bolsa suele durar entre 8 y 24 horas.
Si le administran más CLINIMIX del que debe
Si la dosis administrada es demasiado elevada o la perfusión demasiado rápida, puede hacer que aumente su volumen de circulación sanguínea o que la sangre se vuelva excesivamente ácida. El contenido de glucosa puede aumentar el nivel de glucosa de su sangre y orina. La administración de un volumen excesivo puede producirle náuseas, vómitos, temblores y alteraciones electrolíticas. En estas situaciones, la perfusión debe detenerse inmediatamente.
En algunos casos graves, es posible que su médico deba someterle a una diálisis renal temporal con el objetivo de ayudar a sus riñones a eliminar el exceso de producto.
Para evitar que se produzcan estos casos, su médico supervisará regularmente su estado y analizará sus parámetros sanguíneos.
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran. Si observa algún cambio en la forma de sentirse durante el tratamiento o después de él, comuníqueselo inmediatamente a su médico o enfermero.
Las pruebas que su médico le realizará mientras le administren este medicamento deben minimizar el riesgo de efectos adversos.
La perfusión se detendrá inmediatamente si se desarrolla cualquier signo anormal o síntomas de una reacción alérgica, como presión arterial anormalmente alta o baja, la aparición de una coloración azul o púrpura de la piel, frecuencia cardiaca anormalmente alta, dificultad respiratoria, vómitos, náuseas, erupciones cutáneas, aumento de la temperatura corporal, excesiva sudoración y escalofríos.
Se han observado otros efectos adversos, que se producen con más o menos frecuencia:
- Anafilaxia (una reacción alérgica grave que es de inicio rápido y puede causar la muerte).
- Niveles altos de glucosa, amoníaco y compuestos que contienen nitrógeno en la sangre.
- Deterioro de la función hepática, análisis de sangre de la función hepática anormal.
- Inflamación de la vesícula biliar, presencia de cálculos biliares en la vesícula biliar.
- Inflamación de las venas en el sitio de infusión, irritación venosa, dolor, irritación, ardor, tumefacción.
- Presencia de glucosa en la orina.
- Coma diabético
- Formación de partículas pequeñas que bloquean los vasos sanguíneos pulmonares.
Comunicación de efectos adversos:
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Clinimix
Mantener este medicamento fuera de la vista y del alcance de los niños.
Cuando se utilice en recién nacidos y niños menores de 2 años, la solución (en las bolsas y equipos de administración) se debe proteger de la exposición a la luz hasta que finalice la administración (ver sección 2).
No utilice este medicamento después de la fecha de caducidad que aparece en el envase y el embalaje exterior (MM/AAAA). La fecha de caducidad es el último día del mes que se indica.
No congelar.
Conservar el envase en el embalaje exterior.
No tire los medicamentos por los desagües ni a la basura.. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adiconal
Composición de Clinimix
Los principios activos de cada bolsa de la solución reconstituida son:
Principios activos | 1 l | 1,5 l | 2 l |
L-alanina | 5,70 g | 8,54 g | 11,39 g |
L-arginina | 3,17 g | 4,75 g | 6,33 g |
Glicina | 2,84 g | 4,25 g | 5,67 g |
L-histidina | 1,32 g | 1,98 g | 2,64 g |
L-isoleucina | 1,65 g | 2,48 g | 3,30 g |
L-leucina | 2,01 g | 3,02 g | 4,02 g |
L-lisina (como hidrocloruro de lisina) | 1,60 g (2,00 g) | 2,39 g (2,99 g) | 3,19 g (3,99 g) |
L-metionina | 1,10 g | 1,65 g | 2,20 g |
L-fenilalanina | 1,54 g | 2,31 g | 3,08 g |
L-prolina | 1,87 g | 2,81 g | 3,74 g |
L-serina | 1,38 g | 2,06 g | 2,75 g |
L-treonina | 1,16 g | 1,73 g | 2,31 g |
L-triptófano | 0,50 g | 0,74 g | 0,99 g |
L-tirosina | 0,11 g | 0,17 g | 0,22 g |
L-valina | 1,60 g | 2,39 g | 3,19 g |
Acetato de sodio 3H2O | 2,16 g | 3,23 g | 4,31 g |
Fosfato de potasio dibásico | 2,61 g | 3,92 g | 5,22 g |
Cloruro de sodio | 1,12 g | 1,68 g | 2,24 g |
Cloruro de magnesio 6H2O | 0,51 g | 0,77 g | 1,02 g |
Glucosa anhidra (como glucosa monohidratada) | 75 g (83 g) | 113 g (124 g) | 150 g (165 g) |
Cloruro de calcio 2H2O | 0,33 g | 0,50 g | 0,66 g |
Los demás componentes son:
- ácido acético, ácido clorhídrico (para ajustar el pH de la solución),
- agua para preparaciones inyectables.
Aspecto de CLINIMIX y contenido del envase
CLINIMIX es una solución para perfusión que se presenta en una bolsa de plástico de varias capas y con dos cámaras. El material de la capa interior (de contacto) de la bolsa está fabricado de polímeros (mezcla de copolímeros poliolefínicos) para ser compatible con los componentes y los aditivos autorizados. Otras capas están fabricadas de EVA (polietileno-vinil-acetato) y de un copoliester.
Antes de la reconstitución, las soluciones de glucosa y aminoácidos son transparentes, incoloras o ligeramente amarillentas. Después de la reconstitución, la solución también es transparente, incolora o ligeramente amarillenta.
Para evitar el contacto con el oxígeno del aire la bolsa está envasada en el interior de una sobrebolsa que actúa como barrera de oxígeno, que contiene un absorbente de oxígeno.
Tamaños de envase
Bolsa de 1000 ml: caja de cartón con 8 bolsas
1 bolsa de 1000 ml
Bolsa de 1500 ml: caja de cartón con 6 bolsas
1 bolsa de 1500 ml
Bolsa de 2000 ml: caja de cartón con 4 bolsas
1 bolsa de 2000 ml
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Baxter S.L.
Pouet de Camilo 2,
46394 Ribarroja del Turia (Valencia) España
Responsable de la fabricación
Baxter SA, Boulevard René Branquart, 80, 7860 Lessines, Bélgica
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Clinimix N9G15E, solución para perfusión
En algunos países está registrado con otro nombre, como se indica a continuación
Alemania: Clinimix 3% GE
La última revisión de este prospecto fue en septiembre 2021
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
-------------------------------------------------------------------------------------------------------------------
Esta información está destinada únicamente a profesionales del sector sanitario:
- Composición cuantitativa
Tras mezclar el contenido de los dos compartimentos, la composición de la mezcla binaria para todos los tamaños de bolsa disponibles proporciona lo siguiente:
1 l | 1,5 l | 2 l | |
Nitrógeno (g) Aminoácidos (g) Glucosa (g) | 4,6 28 75 | 6,8 41 113 | 9,1 55 150 |
Calorías totales (kcal) Calorías de glucosa (kcal) | 410 300 | 615 450 | 820 600 |
Sodio (mmol) Potasio (mmol) Magnesio (mmol) Calcio (mmol) | 35 30 2,5 2,3 | 53 45 3,8 3,4 | 70 60 5,0 4,5 |
Acetato (mmol) Cloruro (mmol) Fosfato como HPO4 2- (mmol) | 50 40 15 | 75 60 23 | 100 80 30 |
pH Osmolaridad (mOsm/l) | 6 845 |
- Posología y forma de administración.
Antes de administrar el producto, se debe romper el sello no permanente entre los dos compartimentos y mezclar el contenido de ambos.
Dosis y velocidad de perfusión
La dosis debe individualizarse según los necesidades nutricionales/de líquidos del paciente, del gasto energético, estado clínico, peso corporal y de la capacidad para metabolizar los componentes de Clinimix, así como de la energía o proteínas adicionales administradas por vía oral/enteral. Además, las necesidades diarias de líquidos, nitrógeno y energía decrecen continuamente con la edad.
En adultos, las necesidades oscilan entre 0,16 g de nitrógeno/kg/día (aproximadamente 1 g de aminoácido/kg/día) y 0,32 g de nitrógeno/kg/día (aproximadamente 2 g de aminoácido/kg/día).
En lactantes, las necesidades oscilan entre 0,16 g de nitrógeno/kg/día (aproximadamente 1 g de aminoácido/kg/día) y 0,40 g de nitrógeno/kg/día (aproximadamente 2,5 g de aminoácido/kg/día).
En adultos y pacientes de 12 a 18 años las necesidades de calorías oscilan entre 25 kcal/kg/día y 40 kcal/kg/día, dependiendo del estado de nutrición del paciente y el nivel de catabolismo. Los pacientes menores de 12 años pueden tener requisitos más altos.
Puede haber situaciones clínicas donde los pacientes requieran cantidades de nutrientes que difieran de la composición de Clinimix. En esta situación, cualquier ajuste de volumen (dosis) debe tener en cuenta el efecto resultante que tendrá sobre la dosificación de todos los demás componentes nutriticionales de Clinimix. La velocidad y el volumen de la perfusión deben ser establecidos por un médico prescriptor con experiencia en fluidoterapia intravenosa pediátrica.
Este medicamento no contiene los aminoácidos cisteína y taurina, considerados condicionalmente esenciales para neonatos y lactantes.
Este medicamento no se recomienda para recién nacidos prematuros, a término y para niños menores de 2 años.
La velocidad de administración debe ajustarse en función de la dosis, las características de la solución perfundida, la ingesta total de volumen en 24 horas y la duración de la perfusión.
El tiempo de perfusión debe ser superior a 8 horas. Normalmente, la velocidad de administración se aumenta gradualmente durante la primera hora sin superar los 3 ml por kilogramo de peso corporal por hora, y la dosis máxima es 40 ml por kilogramo de peso corporal al día.
Forma de administración
Cuando se utilice en recién nacidos y niños menores de 2 años, la solución (en las bolsas y equipos de administración) se debe proteger de la exposición a la luz hasta que finalice la administración.
Vía de administración
Se administrará por vía intravenosa periférica o central en función de la osmolaridad final de la mezcla. En general, el límite aceptado para la perfusión periférica es de aproximadamente 800 mOsm/l, pero varía considerablemente en función de la edad, el estado general del paciente y las características de las venas periféricas.
- Advertencias y precauciones especiales de empleo
ADVERTENCIAS
Con formulaciones de CLINIMIX se han notificado reacciones de hipersensibilidad/a la perfusión, incluyendo hipotensión, hipertensión, cianosis periférica, taquicardia, disnea, vómitos, náuseas, urticaria, erupción cutánea, prurito, eritema, hiperhidrosis, fiebre y escalofríos.
Con otros productos de nutrición parenteral se ha notificado anafilaxia.
Al iniciar cualquier perfusión intravenosa es necesaria una monitorización clínica especial. En caso de producirse signo o síntoma anormal, por ejemplo una reacción de hipersensibilidad o reacción a la perfusión, debe interrumpirse la perfusión inmediatamente.
Las soluciones que contienen glucosa se deben usar con precaución, en todo caso, en pacientes con alergia conocida al maíz o productos derivados del maíz.
Se han notificado precipitados vasculares pulmonares en pacientes que reciben nutrición parenteral.
En algunos casos, se han producido resultados mortales. La adición excesiva de calcio y fosfato aumenta el riesgo de la formación de precipitados de fosfato de calcio. Los precipitados se han notificado incluso en ausencia de la sal de fosfato en la solución. También se han notificado precipitación distal en el filtro en línea y se sospecha en la formación de precipitado in vivo.
Si se presentan signos de sufrimiento pulmonar, la perfusión debe interrumpirse e iniciarse una evaluación médica.
Además de la inspección de la solución, el equipo de infusión y el catéter también deben ser comprobados periódicamente buscando precipitados.
En los pacientes mayores de 28 días (incluyendo adultos), no debe administrarse ceftriaxona por vía intravenosa al mismo tiempo que las soluciones que contienen calcio, incluyendo CLINIMIX N9G15E, a través de la misma vía de perfusión. Si se utiliza la misma línea de perfusión para la administración secuencial, debe limpiarse cuidadosamente con un líquido compatible entre las infusiones.
La utilización de catéteres intravenosos para administrar formulaciones parenterales, un mal mantenimiento de los catéteres o las soluciones contaminadas pueden dar lugar a infección y sepsis.
La inmunosupresión y otros factores, como la hiperglucemia, la desnutrición y/o el estado de enfermedad subyacente pueden predisponer a los pacientes a complicaciones infecciosas.
El cuidado sintomático y el control de laboratorio de fiebre/escalofríos, leucocitosis, complicaciones técnicas con el dispositivo de acceso y la hiperglucemia pueden ayudar a reconocer las infecciones tempranas.
La aparición de complicaciones sépticas se puede disminuir haciendo un mayor énfasis en el uso de una técnica aséptica en la colocación del catéter, en su mantenimiento, y en la preparación de la fórmula nutricional.
La realimentación de pacientes gravemente desnutridos puede dar a un síndrome de realimentación, que se caracteriza por el cambio del potasio, fósforo y magnesio intracelular dado que el paciente se encuentra en estado anabólico. También pueden aparecer una deficiencia de tiamina y una retención de líquidos. La supervisión estricta y la ingesta gradual de nutrientes evitando la sobrealimentación pueden prevenir estas complicaciones.
Las soluciones hipertónicas pueden provocar irritación venosa si se perfunden a través de una vena periférica. La elección de una vena periférica o de una vena central depende de la osmolaridad final de la mezcla.
El límite aceptado generalmente para una perfusión periférica es alrededor de 800 mOsm/l, pero varía considerablemente con la edad y estado general del paciente y las características de las venas periféricas.
No conectar en serie envases de plástico con el fin de evitar embolias gaseosas debidas al posible aire residual contenido en el envase primario.
PRECAUCIONES
Antes de iniciar la perfusión, deben corregirse las alteraciones graves en el equilibrio del agua y los electrolitos, los estados graves de sobrecarga de fluidos, y los trastornos metabólicos graves.
Se pueden producir complicaciones metabólicas si la ingesta de nutrientes no se adapta a los requerimientos del paciente, o la capacidad metabólica de cualquier componente alimenticio no es evaluada con precisión. Pueden aparecer efectos metabólicos adversos por la administración inadecuada o excesiva de nutrientes, o por la composición de una mezcla no apropiada para las necesidades específicas del paciente.
Es imprescindible realizar evaluaciones clínicas y determinaciones de laboratorio con frecuencia para el correcto control durante la administración. Éstas incluirán determinación de ionograma y pruebas funcionales del riñón y del hígado.
Deben determinarse y controlarse cuidadosamente las necesidades electrolíticas de los pacientes que reciban estas soluciones, sobre todo en el caso de soluciones sin electrolitos.
La intolerancia a la glucosa es una complicación metabólica común en pacientes gravemente estresados. La perfusión de esta solución puede producir hiperglucemia, glucosuria y síndrome hiperosmolar. La glucosa en sangre y orina debe controlarse de forma rutinaria y si es necesario, para los diabéticos debe adaptarse la dosis de insulina.
Usar con precaución en pacientes con insuficiencia renal, especialmente si hay una hiperpotasemia presente, debido al riesgo de aparición o empeoramiento de la acidosis metabólica e hiperazotemia si no se está realizando la eliminación extra-renal de los desechos. El estado de los líquidos y electrolitos debe ser controlado cuidadosamente en estos pacientes. En caso de insuficiencia renal grave, se deben elegir soluciones de aminoácidos especialmente formuladas.
Se debe tener precaución al administrar Clinimix a los pacientes con insuficiencia suprarrenal.
Se debe evitar la sobrecarga circulatoria especialmente en pacientes con edema pulmonar, insuficiencia y/o fallo cardíaco. El estado de los fluidos debe controlarse cuidadosamente.
Aparte de las pruebas de la función hepática de rutina, en los pacientes con enfermedad hepática preexistente o insuficiencia hepática se deben controlar los posibles síntomas de hiperamonemia.
Es conocido que en algunos pacientes con nutrición parenteral aparecen trastornos hepatobiliares incluyendo colestasis, esteatosis hepática, fibrosis y cirrosis, que pueden dar lugar a insuficiencia hepática, así como colecistitis y colelitiasis. Se cree que la etiología de estos trastornos es multifactorial y puede diferir entre pacientes. Aquellos que desarrollen parámetros de laboratorio anormales u otros signos de trastornos hepatobiliares deben ser evaluados rápidamente por un experto clínico en enfermedades hepáticas con el fin de identificar los posibles factores causales y contribuyentes, y las posibles intervenciones terapéuticas y profilácticas.
En pacientes que reciben soluciones de aminoácidos puede tener lugar un aumento de los niveles de amoniaco en sangre y la hiperamonemia. En algunos pacientes, esto puede indicar la presencia de un trastorno congénito del metabolismo de los aminoácidos (véase la Sección 4. 3 de la Ficha Técnica) o de una insuficiencia hepática.
Se debe medir con frecuencia el amoníaco en sangre en recién nacidos y lactantes para detectar la hiperamonemia, que puede indicar la presencia de una anormalidad congénita del metabolismo de los aminoácidos.
Según el grado y etiología, la hiperamonemia puede requerir una intervención inmediata.
Una perfusión de aminoácidos demasiado rápida puede provocar náuseas, vómitos y escalofríos. En estos casos hay que interrumpir inmediatamente la perfusión.
En general, la dosis para los pacientes ancianos debe ser cautelosa, teniendo en cuenta la mayor frecuencia de insuficiencia hepática, renal o cardíaca y de enfermedades concomitantes o la farmacoterapia.
Población pediátrica
- No se han realizado estudios en la población pediátrica.
- Ver más arriba en relación con el seguimiento de la hiperamonemia en pacientes pediátricos.
La exposición a la luz de las soluciones para nutrición parenteral por vía intravenosa, en especial después de mezclarlas con oligoelementos o vitaminas, puede tener efectos adversos en el desenlace clínico de los recién nacidos debido a la generación de peróxidos y otros productos de degradación. Cuando se utilice en recién nacidos y niños menores de 2 años, Clinimix se debe proteger de la luz ambiental hasta que finalice la administración.
- Información práctica sobre la preparación y el manejo
Precaución: Administre el producto únicamente después de romper el sello y mezclar el contenido de los dos compartimentos
1. |
| 2. |
| 3. |
|
Rompa desde la parte superior para abrir la sobrebolsa. | Retire la parte frontal de la sobrebolsa para acceder a la bolsa de Clinimix. Deseche la sobrebolsa y el sobrecito con el absorbente de oxígeno. | Coloque la bolsa sobre una superficie horizontal y limpia con el asa delante de usted. | |||
4. |
| 5. |
| 6. |
|
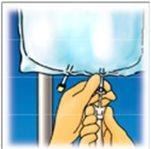
Levante la zona del colgador para retirar la solución de la parte superior de la bolsa. Enrolle firmemente la bolsa hasta que se abra completamente el sello (aproximadamente a la mitad). | Mezcle el contenido invirtiendo la bolsa al menos 3 veces. | Cuelgue la bolsa. Gire el protector para retirarlo delpuerto de administración. Conecte firmemente el conector punzón. |
Use la solución sólo si es transparente, incolora o ligeramente amarillenta, y si el envase no está dañado.
CLINIMIX debe estar a temperatura ambiente antes de su uso.
La activación de CLINIMIX se puede realizar en la sobrebolsa o una vez se haya retirado ésta.
Para un solo uso.
No guarde los envases a medio utilizar y deseche todo el equipo después de utilizarlo.
No vuelva a conectar una bolsa parcialmente utilizada.
No conectar en serie.
Cuando se utilice en recien nacidos y niños menores de 2 años, debe protegerse de la exposición a la luz hasta que finalice la administración. La exposición de Clinimix a la luz ambiental, en especial después de mezclarlo con oligoelementos o vitaminas, genera peróxidos y otros productos de degradación que pueden reducirse si se protege el producto de la exposición a la luz.
Suplementación
Se deberán proporcionar lípidos, vitaminas y oligoelementos a los pacientes que reciban alimentación parenteral durante un largo periodo de tiempo.
Si es necesaria la administración de aditivos, se deberán comprobar las compatibilidades y controlar la estabilidad de las mezclas.
La suplementación puede hacerse después de abrir los sellos no permanentes para todos los aditivos (una vez que se han mezclado las dos soluciones). CLINIMIX puede suplementarse con:
- Emulsiones lipídicas (por ejemplo ClinOleic) a un ritmo de 50 a 250 ml por litro de CLINIMIX
CLINIMIX N9G15E 1 l + 100 ml de Lípidos 20%* | CLINIMIX N9G15E 1,5 l + 100 ml de Lípidos 20%* | CLINIMIX N9G15E 2 l + 250 ml de Lípidos 20%* | |
Nitrógeno (g) Aminoácidos (g) Glucosa (g) Lípidos (g) | 4,6 28 75 20 | 6,8 41 113 20 | 9,1 55 150 50 |
Calorías totales (kcal) Calorías de glucosa (kcal) Calorías lipídicas (kcal) Relación glucosa/lípidos | 610 300 200 60/40 | 815 450 200 69/31 | 1320 600 500 55/45 |
Sodio (mmol) Potasio (mmol) Magnesio (mmol) Calcio (mmol) Acetato (mmol) Cloruro (mmol) Fosfato como HPO4 2- (mmol) | 35 30 2,5 2,3 50 40 15 | 53 45 3,8 3,4 75 60 23 | 70 60 5,0 4,5 100 80 30 |
pH Osmolaridad (mOsm/l) | 6 795 | 6 810 | 6 785 |
- Electrolitos: por litro de CLINIMIX
Hasta una concentración final de | Sodio | Potasio | Magnesio | Calcio |
80 mmol | 60 mmol | 5,6 mmol | 3,0 mmol |
- Oligoelementos: por litro de CLINIMIX
Hasta una concentración final de | Cobre | 10 μmol | Zinc | 77 μmol |
Cromo | 0,14 μmol | Manganeso | 2,5 μmol | |
Flúor | 38 μmol | Cobalto | 0,0125 μmol | |
Selenio | 0,44 μmol | Molibdeno | 0,13 μmol | |
Yodo | 0,5 μmol | Hierro | 10 μmol |
- Vitaminas: por litro de CLINIMIX
Hasta una concentración final de | Vitamina A | 1750 UI | Biotina | 35 μg |
Vitamina B6 | 2,27 mg | Vitamina B1 | 1,76 mg | |
Vitamina D | 110 UI | Ácido fólico | 207 μg | |
Vitamina B12 | 3,0 μg | Vitamina B2 | 2,07 mg | |
Vitamina E | 5,1 mg | Vitamina C | 63 mg | |
Vitamina PP | 23 mg | Vitamina B5 | 8,63 mg | |
Vitamina K | 75 μg |
Datos de estabilidad para la suplementación de CLINIMIX con otras emulsiones lipídicas comercializadas y otros aditivos o nutrientes están disponibles bajo petición.
Si se observa la formación de una ligera crema, mezclar completamente la mezcla mediante una suave agitación para obtener una emulsión uniforme antes de la perfusión.
Las adiciones deben efectuarse en condiciones asépticas.
Las adiciones pueden realizarse con una jeringa o con un equipo de transferencia.
- Adición con jeringa o equipo de transferencia con aguja.
- Preparar el puerto de inyección (el tubo único, ver figura 1).
- Pinchar el tubo e inyectar.
- Mezclar la solución y los aditivos.
Incompatibilidades
Los aditivos pueden ser incompatibles, consulte al fabricante para obtener más detalles.
Si es necesario añadir aditivos, se deben revisar las compatibilidades y controlar la estabilidad de las mezclas.
La solución no debe administrarse con, antes, ni después de una administración de sangre a través del mismo equipo, dada la posibilidad de pseudoaglutinación.
CLINIMIX N9G15E contiene iones de calcio lo que supone un riesgo adicional de coagulación en sangre anticoagulada/conservada con citrato, o sus componentes.
Como con cualquier mezcla de nutrición parenteral, las proporciones de calcio y fosfato deben ser tenidas en cuenta. La adición de un exceso de calcio y fosfato, especialmente en forma de sales minerales, puede dar lugar a la formación de precipitados de fosfato de calcio.
Como en otras soluciones de perfusión que contienen calcio, la administración concomitante de
ceftriaxona y CLINIMIX N9G15E está contraindicada en recién nacidos (≤ 28 días de edad), aunque se utilicen líneas de infusión separadas (riesgo de precipitación mortal de la sal de calcio con ceftriaxona en el torrente sanguíneo del recién nacido).
En pacientes mayores de 28 días (incluyendo adultos), no debe administrarse ceftriaxona por vía intravenosa al mismo tiempo que las soluciones que contienen calcio, incluyendo las soluciones de CLINIMIX N9G15E a través de la misma vía de perfusión (ver sección Advertencias).
Si se utiliza la misma línea de perfusión para la administración secuencial, debe limpiarse cuidadosamente con un líquido compatible entre las infusiones.
- Periodo de validez
2 años si se conserva en la sobrebolsa.
Se recomienda utilizar el producto inmediatamente después de abrir el sello no permanente que hay entre las 2 cámaras. No obstante, una vez reconstituido (es decir, tras abrir el sello interno no permanente), se ha demostrado la estabilidad de la solución reconstituida durante un máximo de 7 días a una temperatura entre 2 °C y 8 °C seguidos de un máximo de 48 h a una temperatura que no supere los 25 °C.
Desde el punto de vista microbiológico, las mezclas deben utilizarse inmediatamente después de realizar las adiciones. Si no se utilizan inmediatamente, el tiempo de conservación en uso y las condiciones antes de su utilización son responsabilidad del usuario, no debiendo ser superior a 24 horas a 2-8ºC, a menos que las adiciones se hubieran realizado en condiciones asépticas controladas y validadas. Si se precisan períodos de conservación más largos en condiciones excepcionales, se puede contactar con la compañía dado que dispone de datos de estabilidad física y química en uso a 7 días a 2-8ºC seguidos de 48 horas a temperatura inferior a 25ºC para los productos indicados en la sección anterior.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a CLINIMIX N9G15E SOLUCION PARA PERFUSIONForma farmacéutica: INYECTABLE PERFUSION, 3,92 g / 1,26 g / 7,21 g / 3,36 g / 4,2 g / 5,11 g / 2,94 g / 2,8 g / 4,76 g / 5,07 g / 4,06 g / 14,49 g / 0,28 g / 8,05 g / 3,5 g / 200 gPrincipio activo: combinationsFabricante: Baxter S.L.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 3,5 g / 200 g / 5,22 g / 1,88 g / 3,92 g / 1,26 g / 7,21 g / 3,36 g / 4,2 g / 5,11 g / 2,94 g / 2,8 g / 662 mg / 1,02 g / 4,76 g / 5,15 g / 5,07 g / 4,06 g / 14,49 g / 0,28 g / 8,05 gPrincipio activo: combinationsFabricante: Baxter S.L.Requiere recetaForma farmacéutica: INYECTABLE PERFUSION, 4,25 g / 300 g / 5,22 g / 1,54 g / 4,76 g / 1,53 g / 8,76 g / 4,08 g / 5,1 g / 6,2 g / 3,57 g / 3,4 g / 662 mg / 1,02 g / 5,78 g / 5,94 g / 6,16 g / 4,93 g / 17,6 g / 0,34 g / 9,78 gPrincipio activo: combinationsFabricante: Baxter S.L.Requiere receta
Médicos online para CLINIMIX N9G15E SOLUCION PARA PERFUSION
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de CLINIMIX N9G15E SOLUCION PARA PERFUSION, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes