
CITICOLINA STADA 1000 MG SOLUCIÓN ORAL EFG

Cómo usar CITICOLINA STADA 1000 MG SOLUCIÓN ORAL EFG
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto:información para el paciente
CiticolinaSTADA1.000 mg solución oral EFG
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento ,porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted , ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico,incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4 .
Contenido del prospecto
- Qué es Citicolina STADA y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Citicolina STADA
- Cómo tomar Citicolina STADA
- Posibles efectos adversos
- Conservación de Citicolina STADA
- Contenido del envase e información adicional
1. Qué es Citicolina STADA y para qué se utiliza
Citicolina STADA pertenece a un grupo de medicamentos llamados psicoestimulantes y nootrópicos, que actúan mejorando el funcionamiento cerebral.
Citicolina STADA se usa para el tratamiento de las alteraciones de la memoria y del comportamiento debidas a:
- un accidente cerebrovascular, que es una interrupción del suministro de sangre en el cerebro por un coágulo o por rotura de un vaso sanguíneo
- un traumatismo craneal, que es un golpe en la cabeza.
2. Qué necesita saber antes de empezar a tomar Citicolina STADA
No tome CiticolinaSTADA
- si es alérgico a citicolina o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- si padece hipertonía del sistema nervioso parasimpático, que es un estado grave con presión arterial baja, sudoración, taquicardia y desmayos.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a tomar citicolina.
- si usted es alérgico al ácido acetilsalicílico, ya que puede provocar asma.
Niños
Citicolina no ha sido adecuadamente estudiada en niños, por lo que sólo debería administrarse si su médico lo considera necesario.
Toma deCiticolinaSTADAcon otros medicamentos
Comunique a su médico o farmacéutico si está tomando , ha tomado recientemente o podría tener que tomar cualquier otro medicamento .
Citicolina potencia los efectos de la L-Dopa, por lo que no se debe administrar a la vez con medicamentos que contengan L-Dopa, sin consultar a su médico. Los medicamentos que contienen L-Dopa habitualmente se utilizan para tratar la enfermedad de Parkinson.
Citicolina no debe administrarse conjuntamente con medicamentos que contengan meclofenoxato, que es un medicamento estimulante cerebral.
Toma de CiticolinaSTADAcon los alimentos y bebidas
Citicolina se puede tomar con las comidas o fuera de ellas.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
Citicolina, al igual que la mayoría de los medicamentos, no se debe administrar si usted está embarazada, si cree que puede estarlo o durante la lactancia a menos que su médico lo considere necesario.
Conducción y uso de máquinas
No se han observado efectos sobre la capacidad de conducir y utilizar maquinaria.
CiticolinaSTADAcontiene sodio, sorbitol, parahidroxibenzoato de propilo, parahidroxibenzoato de metilo y rojo cochinilla
Este medicamento contiene 77 mg de sodio (componente principal de la sal de mesa) por sobre. Esto equivale al 3,85% de la ingesta diaria máxima de sodio recomendada para un adulto.
Este medicamento contiene 2 g de sorbitol por sobre. El sorbitol es una fuente de fructosa. Si su médico le ha indicado que usted (o su hijo) padecen una intolerancia a ciertos azúcares, o se les ha diagnosticado intolerancia hereditaria a la fructosa (IHF), una enfermedad genética rara, en la que el paciente no puede descomponer la fructosa, consulte usted (o su hijo) con su médico antes de tomar este medicamento.
Puede producir reacciones alérgicas (posiblemente retardadas) porque contiene parahidroxibenzoato de propilo (E-217) y parahidroxibenzoato de metilo (E-218).
Este medicamento puede producir reacciones alérgicas porque contiene rojo cochinilla (Ponceau 4R) (E-124). Puede provocar asma, especialmente en pacientes alérgicos al ácido acetilsalicílico.
3. Cómo tomar Citicolina STADA
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico .
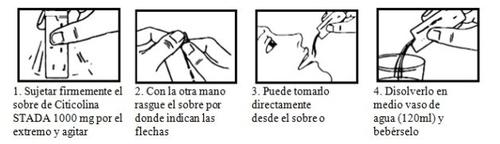
La dosis normal es de 1 a 2 sobres al día, en función de la gravedad de su enfermedad. Puede tomarse directamente o disuelta en medio vaso de agua (120 ml) con las comidas o fuera de ellas.
Si toma más CiticolinaSTADAdel que debe
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico, o llame al Servicio de Información Toxicológica, teléfono: 91 562 04 20, indicando el medicamento y la cantidad ingerida. Se recomienda llevar el envase y el prospecto del medicamento al profesional sanitario.
Si olvidó tomar CiticolinaSTADA
Tome su dosis tan pronto como se acuerde. No tome una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con CiticolinaSTADA
Su médico le indicará la duración de su tratamiento con citicolina. No suspenda el tratamiento antes de consultarlo con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede tener efectos adversos, aunque no todas las personas los sufran.
Los efectos adversos de este medicamento son muy raros (pueden afectar hasta 1 de cada 10.000 pacientes). Puede aparecer dolor de cabeza, vértigo, nauseas, diarrea ocasional, enrojecimiento de la cara, hinchazón de las extremidades y cambios de la presión arterial. Si presenta alguno de estos u otros síntomas, avise a su médico.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Citicolina STADA
Mantener este medicamento fuera de la vista y del alcance de los niños.
Conservar en el envase original.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de "CAD". La fecha de caducidad es el último día del mes que se indica.
No requiere condiciones especiales de conservación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma ayudará a proteger el medioambiente.
6. Contenido del envase e información adicional
Composición de Citicolina STADA
- El principio activo es citicolina. Cada sobre contiene 1.000 mg de citicolina (como sal sódica).
- Los demás componentes (excipientes) son: sacarina sódica, (E-954), Sorbitol líquido (E-420), glicerol (E-422), parahidroxibenzoato de metilo (E-218), parahidroxibenzoato de propilo (E-217), citrato de sodio (E-331), glicerinaformaldehido, sorbato de potasio (E-202), aroma de fresa (contiene propilenglicol (E-1520), color rojo Ponceau 4R (E-124), ácido cítrico (E-330) y agua purificada.
Aspecto del producto y contenido del envase
Citicolina STADA es una solución oral transparente de color rosa, con olor y sabor a fresa envasada en sobre.
Se presenta en envases que contienen 10 o 30 sobres, con 10 ml de solución oral cada uno. Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Laboratorio STADA, S.L.
Frederic Mompou, 5
08960 Sant Just Desvern (Barcelona)
España
Responsable de la fabricación
Galenicum Health, S.L.U.
Sant Gabriel, 50
08950 Esplugues de Llobregat
Barcelona (España)
o
SAG Manufacturing, S.L.U.
Crta N-I, Km 36
28750 San Agustín de Guadalix,
Madrid
Fecha de la última revisión de este prospecto:Diciembre 2013
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS)http://www.aemps.gob.es/
- País de registro
- Precio medio en farmacia16.19 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a CITICOLINA STADA 1000 MG SOLUCIÓN ORAL EFGForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 100 mg/mlPrincipio activo: citicolinaFabricante: Laboratorios Cinfa S.A.Requiere recetaForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 1000 mgPrincipio activo: citicolinaFabricante: Faes Farma S.A.Requiere recetaForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, 1.000 mgPrincipio activo: citicolinaFabricante: Kern Pharma S.L.Requiere receta
Médicos online para CITICOLINA STADA 1000 MG SOLUCIÓN ORAL EFG
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de CITICOLINA STADA 1000 MG SOLUCIÓN ORAL EFG, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes













