
CERDELGA 84 MG CAPSULAS DURAS

Cómo usar CERDELGA 84 MG CAPSULAS DURAS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Cerdelga 21mg cápsulas duras
Cerdelga 84mg cápsulas duras
eliglustat
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener. La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a tomar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Cerdelga y para qué se utiliza
- Qué necesita saber antes de empezar a tomar Cerdelga
- Cómo tomar Cerdelga
- Posibles efectos adversos
- Conservación de Cerdelga
- Contenido del envase e información adicional
1. Qué es Cerdelga y para qué se utiliza
Cerdelga contiene el principio activo eliglustat y se utiliza para el tratamiento prolongado de adultos y niños a partir de 6 años de edad que pesen al menos 15 kg con enfermedad de Gaucher de tipo 1.
Cuando se utiliza en niños, Cerdelga está destinado a aquellos niños cuya enfermedad está bajo control mediante terapia de reemplazo enzimático. El médico determinará si Cerdelga es adecuado para usted o su niño antes de que empiece a tomarlo mediante un sencillo análisis de laboratorio.
La enfermedad de Gaucher de tipo 1 es un trastorno raro hereditario en el cual el cuerpo no descompone correctamente una sustancia llamada glucosilceramida. En consecuencia, la glucosilceramida se acumula en el bazo, el hígado y los huesos. Esta acumulación impide el correcto funcionamiento de estos órganos. Cerdelga contiene el principio activo eliglustat, que reduce la producción de glucosilceramida e impide así su acumulación. A su vez, esto ayuda a los órganos afectados a funcionar mejor.
Hay diferencias entre las personas en la velocidad en que el cuerpo descompone este medicamento. Por tanto, la cantidad de medicamento en la sangre puede diferir de un paciente a otro, lo que puede afectar en cómo un paciente responde al tratamiento. Cerdelga está pensado para usarse en pacientes que descomponen el medicamento a una velocidad normal (lo que se conoce como metabolizadores intermedios y metabolizadores rápidos) o a velocidad baja (lo que se conoce como metabolizadores lentos).
La enfermedad de Gaucher de tipo 1 es una afección que dura toda la vida, por lo que deberá seguir tomando este medicamento según las instrucciones de su médico para obtener el máximo beneficio del tratamiento.
2. Qué necesita saber antes de empezar a tomar Cerdelga
No tome Cerdelga
- Si es alérgico al eliglustat o a alguno de los demás componentes de este medicamento (incluidos en la sección 6).
- Si es un metabolizador intermedio o rápido y usa medicamentos llamados inhibidores potentes o moderados del CYP2D6 (como por ejemplo la quinidina y la terbinafina) usados en combinación con inhibidores potentes o moderados del CYP3A (como por ejemplo la eritromicina y el itraconazol). La combinación de estos medicamentos interferirá en la capacidad de su organismo para descomponer Cerdelga y puede producir niveles más altos del principio activo en la sangre (ver sección “Otros medicamentos y Cerdelga” para obtener una lista ampliada de medicamentos).
- Si es un metabolizador lento y usa medicamentos conocidos como inhibidores potentes del CYP3A (por ejemplo: itraconazol). Los medicamentos de este tipo interferirán con la habilidad de su cuerpo para metabolizar Cerdelga y esto puede resultar en mayores niveles de sustancia activa en su sangre (ver la sección “Otros medicamentos y Cerdelga” para una lista ampliada de medicamentos).
- Si es un metabolizador rápido y tiene la función del hígado disminuida gravemente.
- Si es un metabolizador rápido y tiene la función del hígado disminuida leve o moderadamente mientras toma un inhibidor potente o moderado del CYP2D6.
Advertencias y precauciones
Consulte a su médico o farmacéutico antes de empezar a tomar Cerdelga si:
- está recibiendo tratamiento en estos momentos con alguno de los medicamentos que aparecen en la sección “Otros medicamentos y Cerdelga” o está a punto de hacerlo.
- ha sufrido un infarto de miocardio o insuficiencia cardíaca.
- tiene una frecuencia cardíaca baja.
- tiene un ritmo cardíaco irregular o anómalo, incluida una afección cardíaca llamada síndrome del intervalo QT largo.
- tiene otros problemas del corazón.
- está tomando un medicamento antiarrítmico (que se utiliza para tratar un ritmo cardíaco irregular) como la quinidina, la amiodarona o el sotalol.
- es un metabolizador rápido y tiene la función del hígado disminuida moderadamente.
- es un metabolizador intermedio o lento y tiene la función del hígado disminuida a cualquier nivel.
- es un metabolizador intermedio o lento y tiene la función del riñón disminuida.
- es un paciente con enfermedad renal en etapa terminal (ERET).
Niños y adolescentes
Cerdelga no está destinado para su uso en niños menores de 6 años de edad o que pesen menos de 15 kg.
Otros medicamentos y Cerdelga
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Medicamentos que no se deben tomar combinados entre sí y con Cerdelga
Cerdelga no se debe utilizar con determinado tipo de medicamentos. Estos medicamentos pueden interferir en la capacidad del organismo para descomponer Cerdelga, lo que puede dar lugar a niveles más altos de Cerdelga en la sangre. Estos medicamentos se conocen como inhibidores potentes o moderados del CYP2D6 e inhibidores potentes o moderados del CYP3A. Hay muchos medicamentos en estas categorías y dependiendo de cómo su cuerpo metabolice Cerdelga, los efectos pueden variar de una persona a otra. Consulte a su médico acerca de estos medicamentos antes de empezar a tomar Cerdelga. Su médico determinará qué medicamentos puede utilizar en base a lo rápido que su cuerpo metabolice eliglustat.
Medicamentos que pueden aumentar el nivel de Cerdelga en la sangre:
- paroxetina, fluoxetina, fluvoxamina, duloxetina, bupropión, moclobemida -antidepresivos(usados para tratar la depresión)
- dronedarona, quinidina, verapamilo -antiarrítmicos(usados para tratar el latido cardíaco irregular)
- ciprofloxacino, claritromicina, eritromicina, telitromicina -antibióticos(usados para tratar las infecciones)
- terbinafina, itraconazol, fluconazol, posaconazol, voriconazol -antimicóticos(usados para tratar las infecciones por hongos)
- mirabegrón -usado para tratar la vejiga hiperactiva
- cinacalcet -calcimimético(usado en algunos pacientes con diálisis y en determinados cánceres)
- atazanavir, darunavir, fosamprenavir, indinavir, lopinavir, ritonavir, saquinavir, tipranavir -antirretrovirales(usados para tratar la infección por el VIH)
- cobicistat -utilizado para mejorar los efectos de los antirretrovirales (utilizados para tratar VIH)
- aprepitant -antiemético(usado para reducir los vómitos)
- diltiazem -antihipertensor(usado para aumentar el flujo sanguíneo y reducir la frecuencia cardíaca)
- conivaptán -diurético(usado para elevar los niveles bajos de sodio en la sangre)
- boceprevir, telaprevir – antivirales(usados para tratar Hepatitis C)
- imatinib – anticancerígeno(usado para tratar cáncer)
- amlodipino, ranolazina – usados para tratar la angina de pecho
- cilostazol – usado para tratar dolores tipo calambres en las piernas al caminar, causado por insuficiente suministro sanguíneo en las piernas
- isoniazida – usado para tratar tuberculosis
- cimetidina, ranitidina – antiácidos(usados para tratar la indigestión)
- goldenseal – (también conocido como Hydrastis canadensis), un medicamento a base de plantas obtenido sin receta médica, utilizado para facilitar la digestión.
Medicamentos que pueden reducir el nivel de Cerdelga en la sangre:
- rifampicina, rifabutina -antibióticos(usados para tratar las infecciones)
- carbamazepina, fenobarbital, fenitoína -antiepilépticos(usados para tratar la epilepsia y las convulsiones)
- Hierba de San Juan (también llamada Hypericum perforatum) -un medicamento a base de plantas adquirido sin receta médica que se usa para tratar la depresióny otros trastornos.
Cerdelga puede aumentar el nivel de los tipos siguientes de medicamentos en la sangre:
- dabigatrán -anticoagulante(usado para diluir la sangre)
- fenitoína -antiepiléptico(usado para tratar la epilepsia y las convulsiones)
- nortriptilina, amitriptilina, imipramina, desipramina -antidepresivos(usados para tratar la depresión)
- fenotiazinas -antipsicóticos(usados para tratar la esquizofrenia y la psicosis)
- digoxina -usado para tratar la insuficiencia cardíaca y la fibrilación auricular
- colchicina -usado para tratar la gota
- metoprolol -usado para reducir la presión arterial y/o la frecuencia cardíaca
- dextrometorfano -antitusivo
- atomoxetina -usado para tratar el trastorno por déficit de atención e hiperactividad (TDAH)
- pravastatina -usado para reducir el colesterol y prevenir la enfermedad cardíaca.
Toma de Cerdelga con alimentos y bebidas
Evite el consumo de pomelo o zumo de pomelo, porque puede aumentar el nivel de Cerdelga en la sangre.
Embarazo, lactancia y fertilidad
Si está embarazada, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico, que le dirá si puede tomar este medicamento durante el embarazo.
Se ha demostrado que el principio activo de este medicamento pasa a la leche materna en cantidades muy pequeñas en animales. No se recomienda la lactancia durante el tratamiento con este medicamento. Informe a su médico si está dando el pecho.
No hay efectos conocidos en la fertilidad a dosis normales.
Conducción y uso de máquinas
Cerdelga puede afectar la capacidad para conducir y utilizar máquinas en pacientes que experimenten mareos tras su administración.
Cerdelga contiene lactosa
Si su médico le ha indicado que padece una intolerancia a ciertos azúcares, consulte con él antes de tomar este medicamento.
3. Cómo tomar Cerdelga
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Cerdelga está disponible en 2 dosis diferentes. Las cápsulas duras que contienen 84 mg de eliglustat son de color azul verdoso y blanco, y las cápsulas duras que contienen 21 mg de eliglustat son completamente blancas. Cuando administre este medicamento a su hijo, asegúrese de que está tomando la dosis correcta.
Cerdelga se debe tomar por vía oral en niños que puedan tragar la cápsula entera.
Las cápsulas duras de Cerdelga se deben tomar enteras con agua a la misma hora todos los días. Se pueden tomar con o sin alimentos. Aquellos pacientes que toman la dosis dos veces al día deben tomar una dosis por la mañana y otra dosis por la noche.
No abra, machaque, disuelva ni mastique la cápsula dura antes de tragarla. Si no puede tragar la cápsula entera, informe a su médico.
No se ha estudiado la mezcla del contenido de la cápsula (polvo de eliglustat) con alimentos o bebidas.
Dosis recomendada para adultos
Si es usted metabolizador intermedio o metabolizador rápido:
Trague una cápsula de 84 mg entera dos veces al día con agua. Se puede tomar con o sin alimentos. Tome una cápsula por la mañana y otra por la noche.
Si es usted metabolizador lento:
Trague una cápsula de 84 mg entera una vez al día con agua. Se puede tomar con o sin alimentos. Tome una cápsula al mismo tiempo cada día.
Dosis recomendada para niños
La cantidad de este medicamento que toma su hijo depende de su peso corporal y de cómo metaboliza el medicamento. El médico determinará esto antes de iniciar el tratamiento.
Peso | Si su hijo es metabolizador intermedio o rápido | Si su hijo es metabolizador lento |
Igual o superior a 50 kg | Una cápsula de 84 mg (color azul verdoso y blanco) dos veces al día | Una cápsula de 84 mg (color azul verdoso y blanco) una vez al día |
25 kg a menos de 50 kg | Una cápsula de 84 mg (color azul verdoso y blanco) dos veces al día | Dos cápsulas de 21 mg (color blanco) una vez al día |
15 kg a menos de 25 kg | Dos cápsulas de 21 mg (color blanco) dos veces al día | Una cápsula de 21 mg (color blanco) una vez al día |
Siga tomando Cerdelga todos los días mientras el médico no le indique lo contrario.
Cómo dispensar la cápsula dura de 21 mg
Rompa la lámina que cubre la cápsula con el pulgar o el índice y empuje la cápsula hacia afuera.
Cómo sacar el blíster de la funda para la cápsula dura de 84 mg
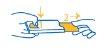
Presionando con el pulgar y el índice juntos en un extremo de la funda (1), tire suavemente del blíster para abrir la funda (2).
Si toma más Cerdelga del que debe
Si toma más cápsulas de las que le indicaron, consulte inmediatamente a su médico. Puede sufrir mareos con pérdida del equilibrio, frecuencia cardíaca baja, náuseas, vómitos y aturdimiento.
Si olvidó tomar Cerdelga
Tome la cápsula siguiente a la hora habitual. No tome una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con Cerdelga
No interrumpa el tratamiento con Cerdelga sin informar a su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Frecuentes(pueden afectar hasta 1 de cada 10 personas):
- Dolor de cabeza
- Mareo
- Cambio en el gusto (disgeusia)
- Palpitaciones
- Irritación de la garganta
- Tos
- Ardor de estómago (dispepsia)
- Dolor de estómago (dolor abdominal superior)
- Diarrea
- Náuseas
- Estreñimiento
- Dolor abdominal
- Enfermedad por reflujo ácido (enfermedad por reflujo gastroesofágico)
- Hinchazón (distensión abdominal)
- Inflamación del estómago (gastritis)
- Dificultad para tragar (disfagia)
- Vómitos
- Boca seca
- Gases (flatulencia)
- Piel seca
- Ronchas (urticaria)
- Dolor de las articulaciones (artralgia)
- Dolor en brazos, piernas o espalda
- Cansancio (fatiga)
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Cerdelga
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase, la funda y el blíster después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
No requiere condiciones especiales de conservación.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Cerdelga
El principio activo es eliglustat (como tartrato).
Cerdelga 21 mg cápsulas duras
Cada cápsula dura contiene 21 mg de eliglustat.
Los demás componentes son:
- En la cápsula: celulosa microcristalina (E460), lactosa monohidrato (ver sección 2 “Cerdelga contiene lactosa”), hipromelosa 15 mPa.S, 2910 y dibehenato de glicerol.
- En la cubierta de la cápsula: gelatina (E441), silicato de potasio y aluminio (E555), dióxido de titanio (E171).
- En la tinta de impresión: shellac, óxido de hierro negro (E172), propilenglicol (E1520) y solución concentrada de amonio (E527).
Cerdelga 84 mg cápsulas duras
Cada cápsula dura contiene 84 mg de eliglustat.
Los demás componentes son:
- En la cápsula: celulosa microcristalina (E460), lactosa monohidrato (ver sección 2 “Cerdelga contiene lactosa”), hipromelosa y dibehenato de glicerol.
- En la cubierta de la cápsula: gelatina (E441), silicato de potasio y aluminio (E555), dióxido de titanio (E171), óxido de hierro amarillo (E172) e indigotina (E132).
- En la tinta de impresión: shellac, óxido de hierro negro (E172), propilenglicol (E1520) y solución concentrada de amonio (E527).
Aspecto de Cerdelga y contenido del envase
Cerdelga 21 mg cápsulas duras
Las cápsulas duras de Cerdelga 21 mg tienen una tapa opaca blanca nacarada y un cuerpo opaco blanco nacarado con la inscripción “GZ04” impresa en negro en la cápsula.
Tamaño de los envases de 56 cápsulas duras en 4 blísteres de 14 cápsulas cada uno.
Cerdelga 84 mg cápsulas duras
Las cápsulas duras de Cerdelga 84 mg tienen una tapa opaca azul verdosa nacarada y un cuerpo opaco blanco nacarado con la inscripción “GZ02” impresa en negro en la cápsula.
Tamaños de los envases de 14 cápsulas duras en 1 blíster, 56 cápsulas duras en 4 blísteres de 14 cápsulas cada uno o 196 cápsulas duras en 14 blísteres de 14 cápsulas cada uno.
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización
Sanofi B.V.
Paasheuvelweg 251105 BP Ámsterdam
Países Bajos
Responsable de la fabricación
Cerdelga 21 mg cápsula dura
Patheon France
40 Boulevard de Champaret
Bourgoin Jallieu
38300
Francia
Cerdelga 84 mg cápsula dura
Sanofi Winthrop Industrie
30-36 avenue Gustave Eiffel
37100 Tours
Francia
Sanofi Winthrop Industrie
1 rue de la Vierge
Ambares et Lagrave
33565 Carbon Blanc cedex
Francia
Genzyme Ireland Ltd
IDA Industrial Park
Old Kilmeaden Road
Waterford
Irlanda
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien/ Luxembourg/Luxemburg Sanofi Belgium Tél/Tel: + 32 2 710 54 00 | Lietuva Swixx Biopharma UAB Tel: +370 5 236 91 40 |
| Magyarország SANOFI-AVENTIS Zrt. Tel.: +36 1 505 0050 |
Ceskárepublika Sanofi s.r.o. Tel: +420 233 086 111 | Malta Sanofi S.r.l. Tel: +39 02 39394275 |
Danmark Sanofi A/S Tlf.: +45 45 16 70 00 | Nederland Sanofi B.V. Tel: +31 20 245 4000 |
Deutschland Sanofi-Aventis Deutschland GmbH Tel.: 0800 04 36 996 Tel. aus dem Ausland: +49 69 305 70 13 | Norge sanofi-aventis Norge AS Tlf: + 47 67 10 71 00 |
Eesti Swixx Biopharma OÜ Tel: +372 640 10 30 | Österreich sanofi-aventis GmbH Tel: + 43 1 80 185 - 0 |
Sanofi-Aventis Μονοπρ?σωπη AEBE +30 210 900 1600 | Polska Sanofi Sp. z o.o. Tel.: +48 22 280 00 00 |
España sanofi-aventis, S.A. Tel: +34 93 485 94 00 | Portugal Sanofi – Produtos Farmacêuticos, Lda. Tel: +351 21 35 89 400 |
France Sanofi Winthrop Industrie Tél: 0 800 222 555 Appel depuis l’étranger: +33 1 57 63 23 23 | România Sanofi Romania SRL Tel: +40 (0) 21 317 31 36 |
Hrvatska Swixx Biopharma d.o.o. Tel: +385 1 2078 500 | Slovenija Swixx Biopharma d.o.o. Tel: +386 1 235 51 00 |
Ireland sanofi-aventis Ireland Ltd. T/A SANOFI Tel: +353 (0) 1 403 56 00 Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Swixx Biopharma s.r.o. Tel: +421 2 208 33 600 |
Italia Sanofi S.r.l. Tel: 800 536 389 | Suomi/Finland Sanofi Oy Puh/Tel: + 358 201 200 300 |
Κ?προς C.A. Papaellinas Ltd. Τηλ: +357 22 741741 | Sverige Sanofi AB Tel: +46 (0)8 634 50 00 |
Latvija Swixx Biopharma SIA Tel: +371 6 616 47 50 |
Fecha de la última revisión de este prospecto:
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: http://www.ema.europa.eu. También existen enlaces a otras páginas web sobre enfermedades raras y medicamentos huérfanos.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a CERDELGA 84 MG CAPSULAS DURASForma farmacéutica: COMPRIMIDO, DesconocidaPrincipio activo: Fenilbutirato sodioFabricante: Immedica Pharma AbRequiere recetaForma farmacéutica: SOLUCIÓN/SUSPENSIÓN ORAL, DesconocidaPrincipio activo: Fenilbutirato sodioFabricante: Immedica Pharma AbRequiere recetaForma farmacéutica: CÁPSULA, 200 mgPrincipio activo: trientineFabricante: Univar Solutions B.V.Requiere receta
Médicos online para CERDELGA 84 MG CAPSULAS DURAS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de CERDELGA 84 MG CAPSULAS DURAS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes










