
CEQUA 0,9 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS

Cómo usar CEQUA 0,9 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Cequa 0,9 mg/ml colirio en solución en envase unidosis
ciclosporina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Cequa y para qué se utiliza
- Qué necesita saber antes de empezar a usar Cequa
- Cómo usar Cequa
- Posibles efectos adversos
- Conservación de Cequa
- Contenido del envase e información adicional
1. Qué es Cequa y para qué se utiliza
Cequa contiene el principio activo ciclosporina. La ciclosporina forma parte de un grupo de medicamentos denominados inmunodepresores, que se utilizan para reducir la inflamación.
Ciclosporina se utiliza para el tratamiento de la enfermedad de ojo seco de moderada a grave (queratoconjuntivitis seca) en pacientes adultos que no han respondido adecuadamente a las lágrimas artificiales.
Debe consultar a un médico si empeora o si no mejora.
Debe acudir a la consulta de su médico como mínimo cada 3 meses para que evalúe el efecto de este medicamento.
2. Qué necesita saber antes de empezar a usar Cequa
No use Cequa:
- si es alérgico a la ciclosporina o a alguno de los demás componentes de este medicamento (incluidos en la sección 6);
- si ha sufrido o sufre cáncer en o alrededor del ojo;
- si sufre una infección ocular.
Advertencias y precauciones
Utilice este medicamento únicamente como colirio para el (los) ojo(s).
Consulte a su médico o farmacéutico antes de empezar a usar este medicamento:
- si ha tenido anteriormente una infección ocular por el virus del herpes que pueda haber dañado la parte frontal transparente del ojo (córnea);
- si está tomando algún medicamento que contenga esteroides;
- si está tomando algún medicamento parat tratar el glaucoma.
Se debe quitar las lentes de contacto antes de usar este medicamento; puede volver a ponérselas 15 minutos después de la administración de este medicamento.
Niños y adolescentes
No se debe usar este medicamento en niños y adolescentes menores de 18 años.
Otros medicamentos y Cequa
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Consulte a su médico si está usando un colirio que contenga esteroides, ya que estos pueden aumentar el riesgo de efectos adversos.
Se debe usar este medicamento al menos 15 minutos después de utilizar cualquier otro colirio.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No debe utilizar este medicamento si está embarazada.
Si se puede quedar embarazada, debe utilizar anticonceptivos mientras use este medicamento.
Es posible que haya cantidades muy pequeñas de ciclosporina en la lecha materna. Si está en periodo de lactancia consulte a su médico antes de utilizar este medicamento.
Conducción y uso de máquinas
Puede que tenga visión borrosa inmediatamente después de utilizar este medicamento. En este caso, espere hasta que su visión sea clara antes de conducir o utilizar máquinas.
Cequa contiene fosfatos
Este medicamento contiene 0,0159 mg de fosfatos en cada gota. Si sufre de daño grave en la capa trasparente de la parte frontal del ojo (córnea), el tratamiento con fosfatos, en casos muy raros, puede provocar parches nublados en la córnea debido al calcio.
3. Cómo usar Cequa
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico o farmacéutico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
La dosis recomendada es una gota en cada ojo afectado, dos veces al día, con un intervalo aproximado de 12 horas.
Instrucciones de uso

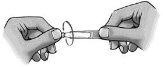
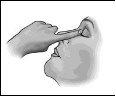
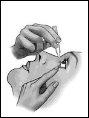 Siga atentamente estas instrucciones y consulte a su médico o farmacéutico si hay algo que no entiende.
Siga atentamente estas instrucciones y consulte a su médico o farmacéutico si hay algo que no entiende.
1 2 3
- Lávese las manos.
- Si tiene puestas las lentes de contacto, quitéselas antes de usar el colirio; puede volvérselas a poner 15 minutos después de haberse administrado las gotas.
- Abra la bolsa de aluminio, extraiga un envase unidosis, e inserte el resto de envases unidosis de nuevo en el interior de la bolsa de aluminio.
- Gire la tapa (figura 1).
- Baje el párpado inferior (figura 2).
- Eche la cabeza hacia atrás y mire al techo.
- Suavemente, apriete para echar una gota de medicamento en el ojo. Asegúrese de no tocar el ojo con la punta del envase unidosis.
- Parpadee varias veces para distribuir la gota en el ojo.
- Después de aplicar la gota, presione con un dedo la comisura del ojo junto a la nariz y cierre suavemente los párpados durante 2 minutos (figura 3)(esto favorece el efecto local).
- Si utiliza el colirio en ambos ojos, repita estos pasos en el otro ojo.
- Tire el envase unidosis en cuanto lo haya utilizado, incluso si aún queda algo de medicamento en él.
- Debe mantener los envases unidosis restantes en la bolsa de aluminio.
Si la gota cae fuera del ojo, inténtelo de nuevo.
Si usa más Cequa del que debe, lávese el ojo con agua. No se aplique más gotas hasta que le corresponda aplicar la siguiente dosis.
En caso de sobredosis o ingestión accidental, consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20 indicando el medicamento y la cantidad ingerida.
Si olvidó usar Cequa,continúe con la siguiente dosis prevista.
No se aplique una dosis doble para compensar las dosis olvidadas. No use más de una gota a la vez en el ojo(s) afectado(s).
Si interrumpe el tratamiento con Cequa, consúltelo antes con su médico.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico o farmacéutico.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Se han observado los siguientes efectos adversos:
Los efectos adversos más frecuentes se producen en y alrededor de los ojos.
Muy frecuentes (pueden afectar a más de 1 de cada 10 personas): dolor o malestar cuando se aplica una gota por primera vez.
Frecuentes (pueden afectar a hasta 1 de cada 10 personas): enrojecimiento del ojo, irritación del ojo, ojos llorosos, irritación en el ojo.
Poco frecuentes (pueden afectar a hasta 1 de cada 100 personas): aumento del lagrimeo del ojo, aumento de la sensibilidad a la luz, dolor en el ojo, hinchazón de la conjuntiva (piel que recubre la parte blanca del ojo), inflamación de los márgenes del párpado, infecciones oculares y perioculares, cefalea e irritación de garganta.
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: www.notificaRAM. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Cequa
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la caja, la bolsa de aluminio y los envases unidosis después de “CAD”. La fecha de caducidad es el último día del mes que se indica.
No conservar por encima de 25°C. No congelar. Después de abrir las bolsas de aluminio, se deben mantener los envases unidosis en ellas para evitar la evaporación.El período de validez tras la apertura de la bolsa de aluminio: 5 días. Desechar inmediatamente después de su uso cualquier envase unidosis individual abierto con restos de solución.
No utilice este medicamento si observa partículas visibles.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de Cequa
- El principio activo es ciclosporina. Un mililitro de Cequa contiene 0,9 mg de ciclosporina.
- Los demás componentes son: macrogolglicerol hidroxiestearato, octoxinol-40, dihidrógenofosfato de sodio dihidrato (E339), hidrogenofosfato de sodio anhidro (E339), cloruro sódico, povidona (E1201), ácido clorhídrico (para ajuste del pH) (E507), hidróxido sódico (para ajuste del pH) (E524) y agua para preparaciones inyectables.
Aspecto del producto y contenido del envase
Cequa es una solución clara, incolora en envases unidosis.
Se suministra en envases unidosis de polietileno de baja densidad (LDPE).
Cada envase unidosis contiene 0,25 ml de colirio en solución.
Los envases unidosis se acondicionan en una bolsa sellada de aluminio con recubrimiento de polietileno.
Tamaños de envases: envases con 60 o 180 envases unidosis, o envase múltiple conteniendo 180 unidades (3 cajas de cartón con 60 envases unidosis cada una).
Puede que solamente estén comercializados algunos tamaños de envases.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Sun Pharmaceutical Industries BV
Polarisavenue 87,
2132JH Hoofddorp,
Países Bajos
Responsable de la fabricación
Laboratoire Unither 1 Rue De L’Arquerie Coutances 50200 Francia |
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Sun Pharma Laboratorios, S.L.
Rambla de Catalunya 53-55
08007 Barcelona
España
Tel: +34 93 342 78 90
Este medicamento está autorizado en los estados miembros del Espacio Económico Europeo con los siguientes nombres:
Holanda: Cequa 0.9 mg/ml oogdruppels, oplossing in verpakking voor eenmalig gebruik
Austria: Cequa 0,9 mg/ml Augentropfen, Lösung im Einzeldosisbehältnis
Alemania: Cequa 0,9 mg/ml Augentropfen, Lösung im Einzeldosisbehältnis
Dinamarca: Sekua 0.9 mg/ml øjendråber, opløsning i enkeltdosisbeholder |
España: Cequa 0,9 mg/ml colirio en solución en envase unidosis
Finlandia: Cequa 0.9 mg/ml silmätipat, liuos kerta- annospakkauksessa | |
Francia: Cequa 0,9 mg/mL, collyre en solution en récipient unidose Italia: Cequa
| |
Suecia: Cequa 0.9 mg/ml ögondroppar, lösning i endosbehållare |
Fecha de la última revisión de este prospecto: Marzo 2024
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) http://www.aemps.gob.es/
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a CEQUA 0,9 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSISForma farmacéutica: COLIRIO, 1 mg/mlPrincipio activo: ciclosporinFabricante: Santen OyRequiere recetaForma farmacéutica: COLIRIO, 1 mg/mlPrincipio activo: ciclosporinFabricante: Santen OyRequiere recetaForma farmacéutica: COLIRIO, 5,5 mg sodio cloruro;3 mg hipomelosa/mlPrincipio activo: artificial tears and other indifferent preparationsFabricante: Alcon Healthcare S.A.No requiere receta
Médicos online para CEQUA 0,9 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de CEQUA 0,9 MG/ML COLIRIO EN SOLUCION EN ENVASE UNIDOSIS, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















