
BILINA 0,5 mg/ml SUSPENSIÓN PARA PULVERIZACIÓN NASAL

Cómo usar BILINA 0,5 mg/ml SUSPENSIÓN PARA PULVERIZACIÓN NASAL
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para elusuario
Bilina0,5 mg/ml suspensión para pulverización nasal
Levocabastina
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o farmacéutico.
- Este medicamento se le ha recetado solamente a usted y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Bilina y para qué se utiliza
- Qué necesita saber antes de empezar a usar Bilina
- Cómo usar Bilina
- Posibles efectos adversos
- Conservación de Bilina
- Contenido del envase e información adicional
1. Qué es Bilina y para qué se utiliza
Bilina contiene levocabastina que es un antihistamínico que se utiliza para el tratamiento sintomático de la rinitis alérgica en adultos y en niños y adolescentes de 4 a menos de 18 años de edad.
La levocabastina es un antagonista altamente selectivo de los receptores H1 de Histamina. Después de la aplicación en la nariz, de una forma casi inmediata y durante horas, se produce el alivio de los síntomas de la rinitis alérgica (estornudos, prurito nasal y rinorrea)..
2. Qué necesita saber antes de empezar a usar Bilina
No use Bilina
- Si es alérgico a levocabastina o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6.1).
Advertencias y precauciones
Si tiene problemas de riñón ya que levocabastina se excreta fundamentalmente por vía renal. Bilina se debe usar con precaución en pacientes con alteraciones renales.
Consulte a su médico o farmacéutico antes de empezar a usar Bilina.
Niños y adolescentes
- No se ha establecido la seguridad y eficacia de este medicamento en niños menores de 4 años.
- Bilina sólo debe usarse para tratar a niños y adolescentes de 4 a menos de 18 años de edad.
Ancianos
Debido a que levocabastina se elimina principalmente por vía renal y a que es habitual una disminución en la funcionalidad renal en los ancianos, se deben tomar precauciones cuando se administre Bilina a este grupo de pacientes.
Uso de Bilina con otros medicamentos
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o podría tener que utilizar cualquier otro medicamento.
Embarazo, lactancia y fertilidad
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, no debe utilizar Bilina, ya que no se han realizado estudios de seguridad en mujeres embarazadas y Bilina pasa a la leche materna. Consulte a su médico o farmacéutico antes de utilizar este medicamento.
Conducción y uso de máquinas
Bilina no produce sedación, ni interfiere con la actividad psicomotora. Sin embargo, si siente sensación de sueño evite conducir o manejar máquinas.
Bilina contiene cloruro de benzalconioy propilenglicol
Este medicamento contiene 0,15 mg de cloruro de benzalconio en cada ml de suspensión para pulverización nasal.
El cloruro de benzalconio puede causar irritación o inflamación dentro de la nariz, especialmente cuando se usa durante periodos largos de tratamiento.
Este medicamento contiene 50 mg de propilenglicol en cada ml de suspensión para pulverización nasal.
3. Cómo usar Bilina
Dosis
Siga exactamente las instrucciones de administración de este medicamento indicadas por su médico. En caso de duda, consulte de nuevo a su médico o farmacéutico.
Adultos y niñosy adolescentesde 4a menos de 18añosde edad
La dosis recomendada es de 2 aplicaciones por fosa nasal, dos veces al día. La dosis se puede aumentar hasta 2 aplicaciones, de 3 a 4 veces al día. El tratamiento debe seguirse hasta que se eliminen los síntomas.
Uso en niñosmenores de 4 años
No se ha establecido la seguridad y eficacia de este medicamento en niños menores de 4 años. No se dispone de datos.
Ancianos
No se dispone de datos de la utilización de levocabastina en ancianos.
Instrucciones de uso
Bilina es una microsuspensión. El envase se debe agitar antes de cada aplicación.
Debe limpiar los conductos nasales antes de administrar el medicamento e inhalarlo por la nariz.
Siga los siguientes pasos:
- Agite el frasco antes de quitar el tapón
- Antes de utilizar Bilina por primera vez, quite el tapón y presione una o dos veces el frasco hasta que se elimine una fina pulverización
- Suénese la nariz
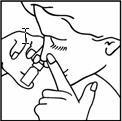
- Incline la cabeza según se muestra en la imagen e introduzca el dosificador del frasco en uno de los orificios de la nariz mientras presiona fuertemente el otro orificio
- Presione dos veces el atomizador en el orificio al mismo tiempo que respira por ese orificio
- Repita los mismo pasos 4 y 5 por el otro orificio.
.
Si usa más Bilina del que debe
Si accidentalmente bebe el contenido de un envase puede sentir sueño. En este caso se recomienda beber abundantemente líquidos no alcohólicos con el fin de acelerar la excreción renal del medicamento y contactar inmediatamente con su médico
En caso de sobredosis o ingestión accidental consulte inmediatamente a su médico o farmacéutico o llame al Servicio de Información Toxicológica, teléfono 91 562 04 20, indicando el medicamento y la cantidad ingerida.
Si olvidó usar Bilina
No use una dosis doble para compensar las dosis olvidadas.
Si tiene cualquier otra duda sobre el uso de este producto, pregunte a su médico o farmacéutico..
4. Posibles efectos adversos
Al igual que todos los medicamentos, Bilina puede producir efectos adversos, aunque no todas las personas los sufran.
La frecuencia de los posibles efectos adversos enumerados a continuación está definida utilizando el siguiente convenio:
Muy frecuentes (puede afectar a más de 1 de cada 10 personas)
Frecuentes (puede afectar hasta 1 de cada 10 personas)
Poco frecuentes (puede afectar hasta 1 de cada 100 personas)
Raros (puede afectar hasta 1 de cada 1.000 personas)
Muy raros (puede afectar hasta 1 de cada 10.000 personas)
Frecuencia no conocida (no puede estimarse a partir de los datos disponibles)
El dolor de cabeza es el efecto adverso más frecuente con Bilina.
Efectos adversos frecuentes (puede afectar hasta 1 de cada 10 personas):
- Náuseas
- Fatiga
- Sinusitis
- Somnolencia
- Mareo
- Dolor de garganta (dolor faringolaríngeo)
- Sangrado de nariz (epistaxis)
- Tos
- Dolor constante
Efectos adversos poco frecuentes (puede afectar hasta 1 de cada 100 personas):
- Irritación, molestia, dolor, quemazón o sequedad en el lugar de la administración
- Nariz congestionada
- Molestias en la nariz
Efectos adversos muy raros (puede afectar hasta 1 de cada 10.000 personas):
- Latidos del corazón rápidos y fuera de lo normal (taquicardia)
- Dificultad respiratoria
- Hipersensibilidad, anafilaxia (Reacciones alérgicas)
- Sensación de malestar general
- Hinchazón de los parpados
- Obstrucción de las vías respiratorias (Broncoespasmo)
- Palpitaciones
Si experimenta efectos adversos, consulte con su médico o farmacéutico, incluso si se trata de efectos adversos que no aparecen en este prospecto.
Comunicación de efectos adversos:
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o farmacéutico, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento..
5. Conservación de Bilina
Mantener fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en el envase después de CAD. La fecha de caducidad es el último día del mes que se indica.
No conservar a temperatura superior a 30ºC.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGREde la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicionall
Composición de Bilina
- El principio activo es levocabastina. Cada mililitro de Bilina contiene 0,5 miligramos de levocabastina.
- Los demás componentes (excipientes) son cloruro de benzalconio, propilenglicol, fosfato de disodio anhidro, fosfato monosódico monohidratado, hipromelosa, polisorbato 80, edetato de disodio y agua purificada.
Aspecto del producto y contenido del envase
Bilina es una suspensión estéril de color blanco que se presenta en envases de plástico.
Cada envase contiene 10 mililitros de suspensión.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Esteve Pharmaceuticals, S.A.
Passeig de la Zona Franca, 109
08038 Barcelona
España
Responsable de la fabricación
Janssen Pharmaceutica, N.V.
Turnhoutseweg, 30
B-2340 Beerse (Bélgica)
Fecha de la última revisión de este prospecto: Diciembre 2021
La información detallada y actualizada de este medicamento está disponible en la página Web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS)http://www.aemps.gob.es/.
- País de registro
- Precio medio en farmacia6.81 EUR
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a BILINA 0,5 mg/ml SUSPENSIÓN PARA PULVERIZACIÓN NASALForma farmacéutica: PRODUCTO USO NASAL, 0,1 g azelastina hidrocloruro/100 mlPrincipio activo: azelastineFabricante: Cooper Consumer Health B.V.Requiere recetaForma farmacéutica: PRODUCTO USO NASAL, 1,5 mg/mlPrincipio activo: azelastineFabricante: Cooper Consumer Health B.V.Requiere recetaForma farmacéutica: PRODUCTO USO NASAL, 1,5 mg/mlPrincipio activo: azelastineFabricante: Cooper Consumer Health B.V.Requiere receta
Médicos online para BILINA 0,5 mg/ml SUSPENSIÓN PARA PULVERIZACIÓN NASAL
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de BILINA 0,5 mg/ml SUSPENSIÓN PARA PULVERIZACIÓN NASAL, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes











