
AMCHAFIBRIN 500 mg SOLUCION INYECTABLE

Cómo usar AMCHAFIBRIN 500 mg SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el paciente
Amchafibrin 500 mg solución inyectable
ácido tranexámico
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico o enfermero.
- Si experimenta efectos adversos, consulte a su médico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- Qué es Amchafibrin 500 mg solución inyectable y para qué se utiliza
- Qué necesita saber antes de empezar a usar Amchafibrin 500 mg solución inyectable
- Cómo usar Amchafibrin 500 mg solución inyectable
- Posibles efectos adversos
- Conservación de Amchafibrin 500 mg solución inyectable
- Contenido del envase e información adicional
1. Qué es Amchafibrin 500 mg solución inyectable y para qué se utiliza
Amchafibrin contiene ácido tranexámico, que pertenece a un grupo de medicamentos denominados antihemorrágicos, antifibrinolíticos, aminoácidos.
Amchafibrin se utiliza en adultos y niños mayores de un año para la prevención y el tratamiento de las pérdidas de sangre debidas a un proceso que inhibe la coagulación de la sangre denominado fibrinólisis.
Las indicaciones específicas incluyen:
- Sangrados menstruales intensos en mujeres
- Sangrado gastrointestinal
- Trastornos hemorrágicos urinarios, tras cirugía de próstata o procedimientos quirúrgicos que afecten al tracto urinario
- Cirugía del oído, nariz y garganta
- Cirugía del corazón, del abdomen o ginecológica
- Sangrado tras haber sido tratado con otro medicamento para disolver los coágulos sanguíneos
2. Qué necesita saber antes de empezar a usar Amchafibrin 500 mg solución inyectable
No useAmchafibrin 500 mgsi:
- Es alérgico al ácido tranexámico o a cualquiera de los demás componentes de este medicamento (incluidos en la sección 6).
- Actualmente sufre una enfermedad que provoque la formación de coágulos sanguíneos
- Sufre un problema llamado ‘coagulopatía de consumo’ en el que la sangre de todo el cuerpo comienza a coagular
- Tiene problemas en los riñones
- Tiene antecedentes de convulsiones
Debido al riesgo de edema cerebral y convulsiones, no se recomienda la inyección intratecal e intraventricular ni la aplicación intracerebral.
Si piensa que cualquiera de las circunstancias anteriores se le aplica o tiene cualquier duda, informe a su médico antes de tomar Amchafibrin 500 mg.
Advertencias y precauciones
Consulte a su médico si cualquiera de estas circunstancias le aplica antes de empezar a usar Amchafibrin 500 mg:
- Si tiene sangre en orina, Amchafibrin 500 mg puede causar obstrucción del tracto urinario.
- Si presenta riesgo de formación de coágulos sanguíneos.
- Si sufre un exceso de formación de coágulos o hemorragias en el cuerpo (coagulación intravascular diseminada), Amchafibrin 500 mg puede no ser adecuado para usted, excepto si padece una hemorragia intensa aguda y los análisis de sangre muestran que se ha activado el proceso que inhibe la coagulación sanguínea llamado fibrinólisis.
- Si ha tenido convulsiones, no se debe administrar Amchafibrin 500 mg. Su médico debe utilizar la menor dosis posible para evitar las convulsiones tras un tratamiento con Amchafibrin 500 mg.
- Si está en tratamiento a largo plazo con Amchafibrin 500 mg, debe prestarse atención a las posibles alteraciones de la visión del color y, si es necesario, el tratamiento debe suspenderse. Con el uso continuo a largo plazo de Amchafibrin 500 mg solución inyectable, está indicado realizar de forma periódica exploraciones oftalmológicas (exámenes de los ojos como agudeza visual, visión de los colores, estudio del fondo de ojo, campo visual, etc.). Ante cambios oftálmicos patológicos, especialmente enfermedades de la retina, su médico debe decidir, después de consultar con un especialista, acerca de la necesidad del uso a largo plazo de la solución inyectable de Amchafibrin 500 mg en su caso.
Otros medicamentos yAmchafibrin 500 mg
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente cualquier otro medicamento, incluso los adquiridos sin receta, vitaminas, minerales, productos de herbolario o suplementos dietéticos.
Concretamente, debe informar a su médico si toma:
- otros medicamentos que ayudan a la sangre a coagular, llamados medicamentos antifibrinolíticos
- medicamentos que impiden la coagulación de la sangre, llamados medicamentos trombolíticos
- anticonceptivos orales. Pueden aumentar el riesgo de que se formen coágulos de sangre.
Embarazo ylactancia
Si está embarazada o en periodo de lactancia, consulte a su médico antes de utilizar Amchafibrin 500 mg.
El ácido tranexámico se excreta en la leche humana. Por tanto, no se recomienda el uso de Amchafibrin 500 mg durante la lactancia.
Conducción y uso de máquinas
No se han realizado estudios sobre la capacidad para conducir y utilizar máquinas.
3. Cómo usar Amchafibrin 500mg
Uso en adultos
Amchafibrin se le administrará mediante inyección lenta en una vena.
Su médico decidirá la dosis correcta para usted y durante cuánto tiempo debe recibirlo.
Uso en niños
Si se administra Amchafibrin a un niño mayor de un año, la dosis debe basarse en el peso del niño. Su médico decidirá la dosis correcta para el niño y durante cuánto tiempo debe recibirlo.
Uso en pacientes de edad avanzada
No es necesaria ninguna disminución de la dosis a menos que haya pruebas de insuficiencia renal.
Uso en pacientes con problemas renales
Si tiene problemas de riñón, la dosis de ácido tranexámico se debe disminuir de acuerdo con los resultados de un análisis de sangre (nivel de creatinina sérica).
Uso en pacientes con insuficiencia hepática
Si tiene problemas de hígado, no es necesaria ninguna disminución de la dosis.
Forma de administración
Amchafibrin solo se debe administrar como inyección intravenosa lenta.
Amchafibrin no se debe inyectar en un músculo.
Si recibe más Amchafibrin 500 mg del que debe
Si ha recibido una dosis mayor de Amchafibrin de la recomendada, podría sufrir una bajada temporal de la presión arterial. Hable inmediatamente con un médico o farmacéutico.
También puede consultar al Servicio de Información Toxicológica. Teléfono 91 562 04 20, indicando el medicamento y la cantidad administrada.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Se han observado los siguientes efectos adversos con Amchafibrin 500 mg:
Frecuentes (pueden afectar hasta 1 de cada 10 pacientes)
- Efectos en estómago e intestino: náuseas, vómitos, diarrea
Poco frecuentes (pueden afectar hasta 1 de cada 100 pacientes)
- Efectos en la piel: erupción
Frecuencia no conocida (la frecuencia no puede estimarse con los datos disponibles)
- Malestar con hipotensión (baja presión arterial), especialmente después de una inyección intravenosa demasiado rápida
- Coágulos en la sangre
- Efectos en el sistema nervioso: convulsiones
- Efectos en los ojos: trastornos de la visión incluyendo el deterioro de la visión del color
- Efectos en el sistema inmunológico: reacciones alérgicas
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del Sistema Español de Farmacovigilancia de Medicamentos de Uso Humano: https://www.notificaram.es. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de Amchafibrin 500 mg
Mantener este medicamento fuera de la vista y del alcance de los niños.
No conservar a temperatura superior a 25ºC. Conservar en el embalaje original.
No utilice Amchafibrin 500 mg inyectable después de la fecha de caducidad que aparece en el cartón después de “CAD” o “EXP”. La fecha de caducidad es el último día del mes que se indica.
Los medicamentos no se deben tirar por los desagües ni a la basura. Deposite los envases y los medicamentos que no necesita en el Punto SIGRE de la farmacia. En caso de duda pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición deAmchafibrin 500 mgsolución inyectable
- El principio activo es ácido tranexámico. Cada ampolla contiene 500 mg de ácido tranexámico.
- Los demás componentes son: agua para inyectables.
AspectoAmchafibrin 500 mgsolución inyectabley contenido del envase
Solución inyectable. Envase conteniendo 6 y 100 ampollas de vidrio de 5 ml.
Titular de la autorización de comercialización y responsable de la fabricación
Titular de la autorización de comercialización
Viatris Healthcare Limited
Damastown Industrial Park
Mulhuddart, Dublín 15
Dublín
Irlanda
Responsable de la fabricación
Biologici Italia Laboratories S.R.L
Via Filippo Serpero,
20060 Masate (MI)
Italia
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
Viatris Pharmaceuticals, S.L.
C/ General Aranaz, 86
28027 - Madrid
España
Fecha de la última revisión de este prospecto:junio 2023
La información detallada de este medicamento está disponible en la página web de la Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) (https://www.aemps.gob.es/)
La siguiente información está destinada únicamente al profesional sanitario:
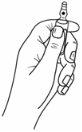
Las ampollas poseen un sistema de apertura OPC (One Point Cut, único punto de apertura) y deben de romperse de acuerdo con las siguientes instrucciones:
- Sostenga con la mano la parte inferior de la ampolla con el pulgar apuntando al punto de color, tal y como se muestra a continuación:
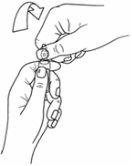
- Agarre la parte superior de la ampolla con la otra mano, apoyando el pulgar en el punto de color y aplicando presión en la dirección opuesta al punto de color tal y como se muestra a continuación:
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a AMCHAFIBRIN 500 mg SOLUCION INYECTABLEForma farmacéutica: COMPRIMIDO, 500 mgPrincipio activo: Tranexamico acidoFabricante: Apc Instytut Sp. Z O.O.Requiere recetaForma farmacéutica: INYECTABLE, 100 mg/mlPrincipio activo: Tranexamico acidoFabricante: Baxter Holding B.V.Requiere recetaForma farmacéutica: COMPRIMIDO, 500 mgPrincipio activo: Tranexamico acidoFabricante: Zentiva K.S.Requiere receta
Médicos online para AMCHAFIBRIN 500 mg SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de AMCHAFIBRIN 500 mg SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes















