ADZYNMA 1.500 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE

Cómo usar ADZYNMA 1.500 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Traducción generada por IA
Este contenido ha sido traducido automáticamente y se ofrece solo con fines informativos. No sustituye la consulta con un profesional sanitario.
Ver originalContenido del prospecto
Introducción
Prospecto: información para el usuario
ADZYNMA 500UI polvo y disolvente para solución inyectable
ADZYNMA 1500UI polvo y disolvente para solución inyectable
rADAMTS13
Este medicamento está sujeto a seguimiento adicional, lo que agilizará la detección de nueva información sobre su seguridad. Puede contribuir comunicando los efectos adversos que pudiera usted tener.La parte final de la sección 4 incluye información sobre cómo comunicar estos efectos adversos.
Lea todo el prospecto detenidamente antes de empezar a usar este medicamento, porque contiene información importante para usted.
- Conserve este prospecto, ya que puede tener que volver a leerlo.
- Si tiene alguna duda, consulte a su médico, farmacéutico o enfermero.
- Este medicamento se le ha recetado solamente a usted, y no debe dárselo a otras personas aunque tengan los mismos síntomas que usted, ya que puede perjudicarles.
- Si experimenta efectos adversos, consulte a su médico, farmacéutico o enfermero, incluso si se trata de efectos adversos que no aparecen en este prospecto. Ver sección 4.
Contenido del prospecto
- 1.Qué es ADZYNMA y para qué se utiliza
- Qué necesita saber antes de empezar a usar ADZYNMA
- Cómo usar ADZYNMA
- Posibles efectos adversos
- Conservación de ADZYNMA
- Contenido del envase e información adicional
- Instrucciones de uso
1. Qué es ADZYNMA y para qué se utiliza
ADZYNMA contiene el principio activo rADAMTS13, una copia sintética de la enzima (proteína) natural ADAMTS13. Las personas con púrpura trombocitopénica trombótica congénita (PTTc) carecen de esta enzima.
La PTT congénita es un trastorno hematológico hereditario muy raro que provoca la formación de coágulos de sangre en los vasos sanguíneos pequeños. Estos coágulos pueden bloquear el flujo de sangre y de oxígeno a los órganos, y esto deriva en un número de plaquetas (componentes de la sangre que contribuyen a su coagulación) inferior al normal en la sangre.
La PTT congénita la provoca una carencia de la enzima ADAMTS13 en la sangre. La ADAMTS13 contribuye a evitar la formación de coágulos de sangre descomponiendo unas moléculas de gran tamaño llamadas factor von Willebrand (VWF). Cuando las moléculas de VWF son demasiado grandes, pueden provocar coágulos de sangre peligrosos. ADZYNMA se utiliza para reponer los niveles de ADAMTS13, lo que ayuda con la descomposición de estas moléculas de gran tamaño en moléculas más pequeñas. Esto reduce la probabilidad de que se formen coágulos de sangre y puede evitar que los niveles de plaquetas de las personas con PTTc sean bajos.
2. Qué necesita saber antes de empezar a usar ADZYNMA
No use ADZYNMA
- Si ha tenido reaciones alérgicas graves o potencialmente mortales a la Radamts13 o alguno de los demás componentes de este medicamento (incluidos en la sección 6).
Advertencias y precauciones
Consulte a su médico, farmacéutico o enfermero antes de empezar a usar ADZYNMA.
Reacciones alérgicas
Existe el riesgo de experimentar una reacción de hipersensibilidad de tipo alérgico con ADZYNMA. Su médico debe informarle acerca de los signos inmediatos de las reacciones alérgicas graves, como:
- frecuencia cardíaca rápida
- opresión en el pecho
- sibilancias y/o aparición repentina de dificultad para respirar
- tensión arterial baja
- urticaria, sarpullido y picor en la piel
- moqueo o congestión nasal
- ojos rojos
- estornudos
- hinchazón rápida debajo de la piel en zonas como cara, garganta, brazos y piernas
- cansancio
- náuseas (sensación de estar enfermo)
- vómitos
- sensación de entumecimiento, hormigueo, agujetas
- inquietud
- anafilaxia (reacción alérgica grave que puede provocar dificultar para tragar y/o respirar y enrojecimiento o hinchazón de la cara y/o de las manos).
Si tiene cualquiera de estos síntomas, su médico decidirá si el tratamiento con ADZYNMA debe interrumpirse y le ofrecerán los medicamentos necesarios para tratar la reacción alérgica. Los síntomas graves, incluidos dificultad respiratoria y mareos, requieren tratamiento urgente.
Inhibidores
Pueden desarrollarse anticuerpos neutralizantes (llamados inhibidores) en algunos pacientes que reciben ADZYNMA. Estos inhibidores podrían potencialmente provocar que el tratamiento deje de funcionar correctamente. Avise a su médico si cree que el medicamento no le está haciendo efecto.
Otros medicamentos y ADZYNMA
Informe a su médico o farmacéutico si está utilizando, ha utilizado recientemente o pudiera tener que utilizar cualquier otro medicamento.
Embarazo y lactancia
Si está embarazada o en periodo de lactancia, cree que podría estar embarazada o tiene intención de quedarse embarazada, consulte a su médico o farmacéutico antes de utilizar este medicamento.
No debe recibir ADZYNMA durante el embarazo a menos que su médico lo recomiende específicamente. Usted y su médico deberán decidir si puede utilizar ADZYNMA durante la lactancia.
Conducción y uso de máquinas
La influencia de este medicamento sobre la capacidad para conducir y utilizar máquinas puede ser pequeña. Tras el uso de ADZYNMA pueden producirse mareo y somnolencia.
Llevar un registro
Con objeto de mejorar la trazabilidad de los medicamentos biológicos, el nombre y el número de lote del medicamento deben estar claramente registrados.
ADZYNMA contiene sodio
Este medicamento contiene menos de 23 mg de sodio (1 mmol) por vial; esto es, esencialmente ”exento de sodio”.
ADZYNMA contiene polisorbato 80
Este medicamento contiene 2,7 mg de polisorbato 80 en cada vial de ADZYNMA 500 UI o 1.500 UI, equivalente hasta 0,216 mg/kg. Los polisorbatos pueden provocar reacciones alérgicas. Informe a su médico si tiene alguna alergia conocida.
3. Cómo usar ADZYNMA
El tratamiento con ADZYNMA se le administrará bajo la supervisión de un médico con experiencia en el tratamiento de pacientes con trastornos de la sangre.
ADZYNMA se administra mediante inyección intravenosa (en una vena). Se proporciona a su médico como un polvo que se disuelve (reconstituye) con el disolvente (un líquido en el que puede disolverse el polvo) suministrado antes de su administración.
La dosis se calcula en función de su peso corporal.
Administración del medicamento en el domicilio
Su médico puede considerar el uso de ADZYNMA en su domicilio si tolera bien las inyecciones. Cuando sea capaz de inyectarse ADZYNMA (o se lo pueda administrar un cuidador) después de recibir la formación pertinente por parte del médico o enfermero responsable del tratamiento, su médico seguirá supervisando su respuesta al tratamiento. Si nota cualquier efecto adverso al utilizar el medicamento en su domicilio, interrumpa inmediatamente la inyección y busque atención de un profesional sanitario.
Dosis recomendada
Terapia de reemplazo enzimática preventiva
La dosis habitual es de 40 UI por kg de peso corporal, administrados cada dos semanas.
Su médico puede cambiar la frecuencia a una vez cada semana, si la administración de ADZYNMA cada dos semanas no le hace efecto.
Terapia de reemplazo enzimática a demanda para episodios repentinos de PTT
Si desarrolla un episodio repentino (agudo) de púrpura trombocitopénica trombótica (PTT), la dosis recomendada de ADZYNMA es la siguiente:
- 40 UI/kg de peso corporal en el día 1.
- 20 UI/kg de peso corporal en el día 2.
- 15 UI/kg de peso corporal una vez al día, empezando en el día 3 y hasta dos días después de que se resuelva el episodio repentino de PTT.
Si usa más ADZYNMA del que debe
Usar demasiada cantidad de este medicamento puede provocar sangrado.
Si olvidó usar ADZYNMA
Si ha omitido una inyección de ADZYNMA, informe a su médico lo antes posible. No tome una dosis doble para compensar las dosis olvidadas.
Si interrumpe el tratamiento con ADZYNMA
Si desea interrumpir el tratamiento con ADZYNMA, consulte a su médico. Si interrumpe el tratamiento, los síntomas de la enfermedad pueden empeorar.
Si tiene cualquier otra duda sobre el uso de este medicamento, pregunte a su médico, farmacéutico o enfermero.
4. Posibles efectos adversos
Al igual que todos los medicamentos, este medicamento puede producir efectos adversos, aunque no todas las personas los sufran.
Se han notificado los siguientes efectos adversos con ADZYNMA:
Muy frecuentes(pueden afectar a más de 1 de cada 10 personas)
- infección nasal y de garganta
- dolor de cabeza
- sensación de mareo
- migraña
- diarrea
- náuseas
Frecuentes(pueden afectar hasta a 1 de cada 10 personas)
- número elevado de plaquetas en la sangre (trombocitosis)
- somnolencia
- estreñimiento
- hinchazón (distensión abdominal)
- debilidad (astenia)
- sensación de calor
- actividad de ADAMTS13 anormal
Comunicación de efectos adversos
Si experimenta cualquier tipo de efecto adverso, consulte a su médico, farmacéutico o enfermero, incluso si se trata de posibles efectos adversos que no aparecen en este prospecto. También puede comunicarlos directamente a través del sistema nacional de notificación incluido en el Apéndice V. Mediante la comunicación de efectos adversos usted puede contribuir a proporcionar más información sobre la seguridad de este medicamento.
5. Conservación de ADZYNMA
Mantener este medicamento fuera de la vista y del alcance de los niños.
No utilice este medicamento después de la fecha de caducidad que aparece en la etiqueta y en la caja después de CAD. La fecha de caducidad es el último día del mes que se indica.
Viales sin abrir
Conservar en nevera (entre 2 ºC – 8 ºC).
No congelar.
Conservar en el embalaje original para protegerlo de la luz.
Los viales de polvo de ADZYNMA sin abrir pueden almacenarse a temperatura ambiente (hasta 30 °C) durante un periodo de hasta 6 meses, pero sin superar la fecha de caducidad. No volver a meter ADZYNMA en la nevera después de su conservación a temperatura ambiente. Registrar en la caja la fecha en la que se extrajo ADZYNMA de la nevera.
Después de la reconstitución
Desechar el medicamento reconstituido no utilizado una vez transcurridas 3 horas.
No utilice este medicamento si observa que no es transparente e incoloro.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
6. Contenido del envase e información adicional
Composición de ADZYNMA
- El principio activo, ADAMTS13, es una “desintegrina A y metaloproteinasa con motivos de trombospondina 13” recombinante humana purificada.
El contenido nominal de cada vial de polvo es de 500 o 1 500 UI de actividad rADAMTS13.
- El vial de disolvente contiene 5 ml de agua para preparaciones inyectables.
- Los demás excipientes son cloruro de sodio, cloruro de calcio dihidrato, L‑histidina, manitol, sacarosa y polisorbato 80 (E433). Ver sección 2 “ADZYNMA contiene sodio” y “ADZYNMA contiene polisorbato 80”.
Aspecto del producto y contenido del envase
ADZYNMA se suministra como polvo y disolvente para solución inyectable. El polvo es un polvo blanco liofilizado. El disolvente es transparente e incoloro.
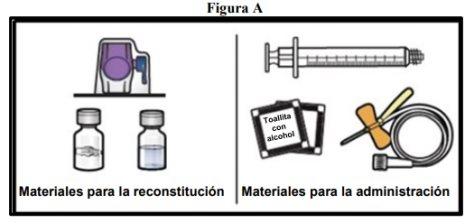
Cada envase contiene un vial de polvo, un vial de disolvente, un dispositivo para la reconstitución (BAXJECT II Hi‑Flow), una jeringa desechable, un equipo de perfusión y dos toallitas con alcohol.
Titular de la autorización de comercialización y responsable de la fabricación
Takeda Manufacturing Austria AG
Industriestrasse 67
1221 Viena
Austria
Pueden solicitar más información respecto a este medicamento dirigiéndose al representante local del titular de la autorización de comercialización:
België/Belgique/Belgien Takeda Belgium NV Tél/Tel: +32 2 464 06 11 | Lietuva Takeda, UAB Tel: +370 521 09 070 |
| Luxembourg/Luxemburg Takeda Belgium NV Tél/Tel: +32 2 464 06 11 |
Ceská republika Takeda Pharmaceuticals Czech Republic s.r.o. Tel: +420 234 722 722 | Magyarország Takeda Pharma Kft. Tel.: +36 1 270 7030 |
Danmark Takeda Pharma A/S Tlf: +45 46 77 10 10 | Malta Τakeda HELLAS S.A. Tel: +30 210 6387800 |
Deutschland Takeda GmbH Tel: +49 (0)800 825 3325 | Nederland Takeda Nederland B.V. Tel: +31 20 203 5492 |
Eesti Takeda Pharma OÜ Tel: +372 6177 669 | Norge Takeda AS Tlf: +47 800 800 30 |
| Österreich Takeda Pharma Ges.m.b.H. Tel: +43 (0) 800-20 80 50 |
España Takeda Farmacéutica España, S.A. Tel: +34 917 90 42 22 | Polska Takeda Pharma Sp. z o.o. Tel.: +48223062447 |
France Takeda France SAS Tél: + 33 1 40 67 33 00 | Portugal Takeda Farmacêuticos Portugal, Lda. Tel: + 351 21 120 1457 |
Hrvatska Takeda Pharmaceuticals Croatia d.o.o. Tel: +385 1 377 88 96 | România Takeda Pharmaceuticals SRL Tel: +40 21 335 03 91 |
Ireland Takeda Products Ireland Ltd Tel: 1800 937 970 | Slovenija Takeda Pharmaceuticals farmacevtska družba d.o.o. Tel: + 386 (0) 59 082 480 |
Ísland Vistor hf. Sími: +354 535 7000 | Slovenská republika Takeda Pharmaceuticals Slovakia s.r.o. el: +421 (2) 20 602 600 |
Italia Takeda Italia S.p.A. Tel: +39 06 502601 | Suomi/Finland Teva Finland Oy Puh/Tel: +358 20 180 5900 |
| Sverige Takeda Pharma AB Tel: 020 795 079 |
Latvija Takeda Latvia SIA Tel: +371 67840082 |
Fecha de la última revisión de este prospecto: 08/2024.
Este medicamento se ha autorizado en «circunstancias excepcionales». Esta modalidad de aprobación significa que debido a la rareza de esta enfermedad no ha sido posible obtener información completa de este medicamento.
La Agencia Europea de Medicamentos revisará anualmente la información nueva de este medicamento que pueda estar disponible y este prospecto se actualizará cuando sea necesario.
Otras fuentes de información
La información detallada de este medicamento está disponible en la página web de la Agencia Europea de Medicamentos: https://www.ema.europa.eu. También existen enlaces a otras páginas web sobre enfermedades raras y medicamentos huérfanos.
- Instrucciones de uso
Estas instrucciones contienen información sobre cómo reconstituir e infundir ADZYNMA.
Estas instrucciones de uso están dirigidas a profesionales sanitarios y a los pacientes/cuidadores que administren ADZYNMA en el domicilio después de recibir la formación adecuada por parte de un profesional sanitario.
El tratamiento con ADZYNMA debe prescribirlo y supervisarlo un profesional sanitario con experiencia en el tratamiento de pacientes con trastornos de la sangre.
Importante:
- Solo para inyección intravenosa después de la reconstitución.
- Use una técnica aséptica durante todo el procedimiento.
- Compruebe la fecha de caducidad del producto antes de utilizarlo.
- Noutilice ADZYNMA si la fecha de caducidad ha pasado.
- Si el paciente necesita más de un vial de ADZYNMA por inyección, reconstituya cada vial de acuerdo con las instrucciones detalladas que se indican en “Reconstitución”.
- Inspeccione la solución reconstituida de ADZYNMA para detectar si hay partículas o cambios de color antes de la administración. La solución debe tener un aspecto transparente e incoloro.
- Nola administre si observa partículas o cambios en el color.
- Si se conserva a temperatura ambiente, use ADZYNMA antes de que transcurran 3horasdesde la reconstitución.
- Noadministre ADZYNMA en el mismo tubo o recipiente y de forma simultánea con otros medicamentos para perfusión.
Reconstitución
- Prepare una superficie limpia y plana y reúna todos los materiales necesarios para la reconstitución y la administración (FiguraA).
|
- Deje que los viales de ADZYNMA y de disolvente alcancen la temperatura ambiente antes de su uso.
- Lávese y séquese las manos concienzudamente.
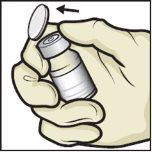
- Retire las tapas de plástico de los viales de ADZYNMA y de disolvente y coloque los viales en una superficie plana (FiguraB).
FiguraB
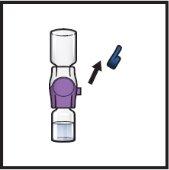
- Limpie los tapones de goma con una toallita con alcohol y deje que se sequen antes de su uso (FiguraC).
FiguraC
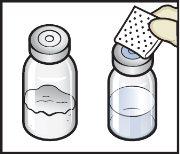
- Abra el envase del dispositivo BAXJECT II Hi‑Flow retirando la tapa, sin tocar el interior (FiguraD).
- Nosaque el dispositivo BAXJECT II Hi‑Flow del envase.
- Notoque la punta de plástico transparente.
FiguraD
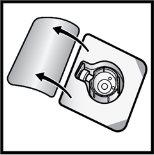
- Gire hacia abajo el envase con el BAXJECT II Hi‑Flow en el interior y colóquelo sobre la parte superior del vial de disolvente. Presione recto hacia abajo hasta que la punta de plástico transparenteperfore el tapón del vial de disolvente(FiguraE).
FiguraE
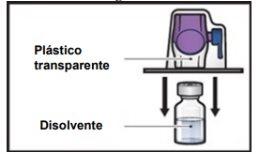
- Sujete el envase del dispositivo BAXJECT II Hi‑Flow por el borde y retírelo del dispositivo (FiguraF).
- Noquite el tapón azuldel dispositivo BAXJECT II Hi‑Flow.
- Notoque la punta de plástico moradaque ha quedado expuesta.
FiguraF
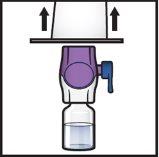
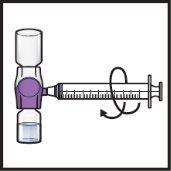
- Dé la vuelta al sistemapara que el vial de disolventequede arriba. Presione el dispositivo BAXJECT II Hi‑Flow recto hacia abajo hasta que la punta de plástico moradaperfore el tapón del vial de polvode ADZYNMA (FiguraG). El vacío hará que el disolvente penetre en el vial de polvode ADZYNMA.
- Es posible que observe burbujas o espuma; esto es normal y deberían desaparecer pronto.
FiguraG
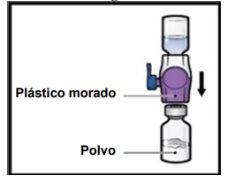
- Gire suavey continuamente los viales conectados haciendo círculos hasta que el polvo se disuelva por completo (FiguraH).
- Noagite el vial.
FiguraH
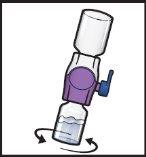
- Antes de la administración, inspeccione visualmente la solución reconstituida para comprobar si tiene partículas.
- Nouse el producto si observa partículas o cambios en el color.
- Si la dosis requiere más de un vial de ADZYNMA, reconstituya cada vial siguiendo los pasos anteriores.
- Utilice un dispositivo BAXJECTII Hi‑Flow diferente para reconstituir cada vial de ADZYNMA.
Administración de ADZYNMA
- Quite el tapón azuldel dispositivo BAXJECT II Hi‑Flow (FiguraI). Conecte una jeringa con conexión Luer‑Lock (FiguraJ).
- Noinyecte aire en el sistema.
|
- Dé la vuelta al sistema(de modo que quede arriba el vial con ADZYNMA). Extraiga la solución reconstituidaen la jeringa tirando lentamente del émbolo (FiguraK).
FiguraK
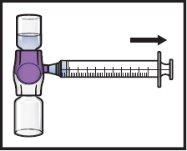
- Si un paciente va a recibir más de un vial de ADZYNMA, puede extraerse el contenido de varios viales en la misma jeringa. Repita este proceso con todos los viales reconstituidos de ADZYNMA hasta alcanzar el volumen total de administración.
- Desconecte la jeringa y conecte una aguja de inyección adecuada o un equipo de perfusión.
- Apunte la aguja hacia arriba y elimine las burbujas de aire dando golpes suaves con el dedo en la jeringa y expulsando lenta y cuidadosamente el aire de la jeringa y de la aguja.
- Aplique un torniquete y limpie el lugar de inyección elegido con una toallita con alcohol (FiguraL).
FiguraL
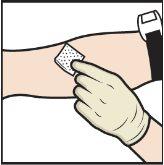
- Inserte la aguja en la vena y retire el torniquete.
- Infunda ADZYNMA reconstituido lentamente, a una velocidad de 2a4ml por minuto(FiguraM).
- Para controlar la velocidad de administración puede utilizarse una bomba de jeringa.
FiguraM
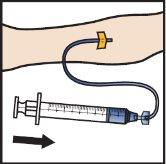
- Saque la aguja de la vena y aplique presión en el lugar de inyección durante varios minutos.
- Novuelva a tapar la aguja.
Conservación de ADZYNMA
- Conservar ADZYNMA en nevera (entre 2 °C - 8 °C) o a temperatura ambiente (hasta 30 °C) durante un periodo de hasta 6 meses.
- Novolver a introducir ADZYNMA en la nevera después de su conservación a temperatura ambiente.
- Registraren la caja la fecha en la que se extrajo ADZYNMA de la nevera.
- Nocongelar.
- Conservar en el embalaje original para protegerlo de la luz.
- Noutilizar después de la fecha de caducidad que aparece en la etiqueta y en la caja después de CAD.
- Usar ADZYNMA antes de que transcurran 3horasdesde la reconstitución. Desechar el medicamento reconstituido que no se haya utilizado antes de que transcurran 3 horas desde la reconstitución.
Eliminación de ADZYNMA
- Los viales son de un solo uso.
- Deseche la aguja usada, la jeringa y los viales vacíos en un recipiente para objetos punzocortantes resistente a perforaciones.
Los medicamentos no se deben tirar por los desagües ni a la basura. Pregunte a su farmacéutico cómo deshacerse de los envases y de los medicamentos que ya no necesita. De esta forma, ayudará a proteger el medio ambiente.
- País de registro
- Principio activo
- Requiere recetaSí
- Fabricante
- Esta información es de carácter general y no sustituye la consulta con un profesional sanitario.
- Alternativas a ADZYNMA 1.500 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLEForma farmacéutica: INYECTABLE, 500 UIPrincipio activo: apadamtase alfa and cinaxadamtase alfaFabricante: Takeda Manufacturing Austria AgRequiere recetaForma farmacéutica: INYECTABLE, 10 mgPrincipio activo: AlteplasaFabricante: Boehringer Ingelheim International GmbhRequiere recetaForma farmacéutica: INYECTABLEPrincipio activo: Proteina cFabricante: Takeda Manufacturing Austria AgRequiere receta
Médicos online para ADZYNMA 1.500 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE
Comenta la dosis, los posibles efectos secundarios, interacciones, contraindicaciones o la revisión de receta de ADZYNMA 1.500 UI POLVO Y DISOLVENTE PARA SOLUCION INYECTABLE, sujeto a valoración médica y a la normativa local.
Preguntas frecuentes