
ZOMACTON 10 mg/ml POWDER AND SOLVENT FOR INJECTION

Ask a doctor about a prescription for ZOMACTON 10 mg/ml POWDER AND SOLVENT FOR INJECTION

How to use ZOMACTON 10 mg/ml POWDER AND SOLVENT FOR INJECTION
Introduction
Package Leaflet: Information for the User
Zomacton 10 mg/ml powder and solvent for solution for injection
Somatropin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours. If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information
- What is Zomacton and what is it used for
- What you need to know before you use Zomacton
- How to use Zomacton
- Possible side effects
- Storage of Zomacton
- Contents of the pack and further information
The information in this leaflet refers to you or to a child or adolescent in your care.
1. What is Zomacton and what is it used for
Zomacton contains the active substance somatropin, also known as growth hormone. Growth hormone is produced naturally in the body. It has a very important function in growth. Zomacton contains somatropin obtained in a pharmaceutical manufacturing facility.
Zomacton is used for the long-term treatment of:
- Growth problems due to the lack of growth hormone in children;
- Growth problems due to Turner Syndrome (a genetic disorder that affects women).
2. What you need to know before you use Zomacton
Do not use Zomacton
- In children when bone growth is complete (epiphyses closed)
- Do not use Zomacton and inform your doctor if you have an active tumor (cancer). Tumors must remain inactive and anti-tumor treatment must have been completed before starting treatment with Zomacton
- If you are allergic to any of the components of Zomacton or to any of the ingredients of this medicine (listed in section 6).
- If you are seriously ill due to the following complications: open heart or abdominal surgery, multiple injuries from an accident, or respiratory failure
- In children with chronic kidney disease after kidney transplant
Warnings and precautions
Consult your doctor or pharmacist before starting to use Zomacton.
Treatment with Zomacton should only be started under the supervision of a specialist doctor, with experience in the diagnosis of patients with growth hormone deficiency.
- Zomacton contains a preservative called metacresol. In very rare cases, the presence of metacresol can cause inflammation (swelling) of the muscles. If you experience muscle pain or pain at the injection site, inform your doctor.
- Patient with Prader-Willi syndrome should not be treated with Zomacton unless they also have growth hormone deficiency.
- If you have a family history of diabetes mellitus, your blood sugar levels should be regularly checked by your doctor.
- If you are diabetic, you will require strict control of blood sugar and your dose will need to be adjusted to maintain diabetic control. Your doctor will inform you if necessary.
- If your growth hormone deficiency is caused by a problem in your brain (intracranial lesion), you should be frequently examined to evaluate possible worsening or recurrence of the disease. If this is confirmed, your doctor will inform you if you need to interrupt treatment with Zomacton.
- If you have had a serious illness, such as cancer, treatment with Zomacton may cause the illness to recur or worsen. Therefore, if you experience any symptoms that concern you, you should consult your doctor immediately.
- If you are receiving glucocorticoid replacement therapy, you should consult your doctor regularly as your glucocorticoid dose may need to be adjusted.
- Treatment with Zomacton may lead to a deficiency of thyroid hormone that may require treatment. To control this, your doctor will carry out periodic checks to ensure that your thyroid gland is functioning correctly.
- Some children with growth hormone deficiency have developed leukemia (an increase in the number of white blood cells in the blood), having received or not received growth hormone treatment. However, there is no evidence that the incidence of leukemia is increased in patients without risk factors treated with growth hormone. No cause-and-effect relationship with growth hormone treatment has been proven
- If you suffer from complications after surgery, trauma, or acute respiratory failure.
- If you require surgery, are seriously injured in an accident, or are seriously ill, your doctor will review your treatment.
- Pancreatitis should be considered in children treated with somatropin who develop abdominal pain.
- Zomacton may cause pancreatitis, which causes severe pain in the abdomen and back. If you or your child develop stomach pain after administering Zomacton, consult your doctor.
If you develop any of the following while being treated with Zomacton, contact your doctor or nearest emergency department urgently:
- repeated or severe headaches
- vision problems
- nausea and/or vomiting
Please consult your doctor immediately if you develop a limp or pain in the hip or knee.
Use in athletes
This medicine contains somatropin that may produce a positive result in doping tests.
Use of other medicines and Zomacton
Tell your doctor or pharmacist:
- If you are being treated with steroids due to insufficient production of ACTH (adrenocorticotropic hormone). This is because the dose of steroids should normally be adjusted while being treated with Zomacton.
- If you are being treated with high doses of androgens, estrogens, and anabolic steroids as they may reduce the increase in final height.
- If you are being treated with medication prescribed regularly, for example steroids, medication for epilepsy, or medication for immune system suppression.
- If you are being treated with insulin, your dose may need to be adjusted to maintain diabetic control. Your doctor will inform you if necessary.
Tell your doctor or pharmacist if you are using or have recently used other medicines, including those obtained without a prescription.
Pregnancy and breastfeeding
Zomacton should not be used during pregnancy or breastfeeding.
Consult your doctor or pharmacist before using any medicine.
Driving and using machines
Zomacton has no influence on the ability to drive or use machines.
3. How to use Zomacton
Follow the instructions for administration of Zomacton exactly as indicated by your doctor. Consult your doctor or pharmacist if you have any doubts.
Your doctor or nurse will inform you of the correct dose for you. The dose is administered by subcutaneous injection (under the skin) with a syringe.
Posology:
Growth hormone deficiency in children:
Your doctor will calculate the exact dose for you, based on your body weight in kilograms (kg). Generally, a dose of 0.17 – 0.23 mg per kg body weight per week is recommended. This weekly amount can be divided into six or seven doses, corresponding to a daily injection of 0.02 – 0.03 mg per kg body weight. The maximum recommended weekly dose is 0.27 mg per kg body weight, equivalent to daily injections of up to approximately 0.04 mg per kg body weight.
Turner Syndrome (only women)
Your doctor will calculate the exact dose for you, based on your body weight. A dose of 0.33 mg per kg body weight per week is generally recommended. This weekly amount can be divided into six or seven doses, equivalent to a daily dose of 0.05 mg per kg body weight.
Reconstitution instructions
Zomacton is provided as a powder and should only be mixed with the solvent (liquid) provided.
The 10 mg/ml solution for injection is prepared by mixing the Zomacton powder with 1 ml of solvent using a pre-filled syringe as described below.
- You should wash your hands.
- Remove the yellow plastic protective cap from the Zomacton vial.
- The top of the vial should be cleaned with an antiseptic solution or with alcohol to avoid contamination of the contents. Do not touch the rubber stopper of the vial after cleaning.
- Take the pre-filled syringe with solvent. Remove the gray cap. Attach the reconstitution needle to the pre-filled syringe. Remove the needle cap.
- Place the needle in the center of the rubber stopper of the cleaned vial and inside the vial, and slowly inject the solvent into the vial, directing the stream of liquid against the glass wall to avoid foaming.
- Discard the syringe in a puncture-proof container.
- Gently turn the vial a few times until the contents are completely dissolved. Do not shake.
- If the solution is cloudy or contains particles, it should not be used. In case of cloudiness after refrigeration, the product should be allowed to reach room temperature. If cloudiness persists, discard the vial and its contents.
The contents should be clear and colorless after reconstitution.



Reconstitution with a normal syringe
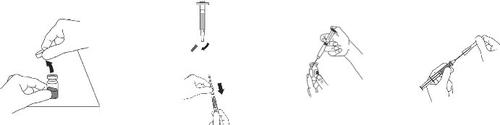
Step 1 | Step 2 | Step 3 | Step 4 |
Remove the yellow cap from the Zomacton vial. | Remove the gray cap from the pre-filled syringe. Attach the reconstitution needle. | Place the needle in the rubber stopper and inside the vial, and slowly inject the solvent into the vial, directing the stream of liquid against the glass wall to avoid foaming. | Replace the needle cap and discard the syringe. |
Step 5.
The vial should then be gently turned until the powder is completely dissolved, forming a clear and colorless solution.
Place the reconstituted Zomacton vial in a vertical position in the refrigerator at 2°C to 8°C.
Avoid shaking or vigorously mixing. If the solution remains cloudy or contains particles, the vial and its contents should be discarded. In case of cloudiness after refrigeration, the solution should be allowed to reach room temperature. If cloudiness persists, discard the vial and its contents.
Administration
The required dose of Zomacton 10 mg/ml is administered using a conventional syringe as shown by your healthcare professional in the clinic.
After reconstitution, the following steps should be performed for injection.
- You should wash your hands
- The top of the vial should be cleaned with an alcohol swab to avoid contamination of its contents. Do not touch the rubber stopper after cleaning
- Hold the vial upside down, keeping the top of the needle below the surface of the medication. Gently pull the plunger until the prescribed amount of medication fills the syringe. If you do not have enough medication for a full dose, reconstitute a new vial to make up the difference.
- With the needle still in the inverted vial, gently tap the syringe to loosen any air bubbles.
- Remove the needle from the vial and carefully replace the needle cap until you are ready to inject.
- Thoroughly clean the injection site with alcohol.
- Check that the syringe contains the correct dose.
- Remove the needle cap and hold the syringe in the same way you hold a pencil.
- With your free hand, gently pinch the skin around the injection site between your fingers.
- Insert the needle into the tissue under the skin surface at an angle of 45° to 90° to reduce discomfort.
- Holding the syringe in place, slowly push the plunger until the syringe is empty
- Quickly withdraw the needle and apply pressure to the injection site with a sterile swab. Discard the needle and syringe in a puncture-proof container.
Do not share your syringes, needles, or vials with anyone else. You may infect them or get an infection from them. Any unused product or waste material should be disposed of in accordance with local requirements.
Do not share your syringes, needles, or vials with anyone else. You may infect them or get an infection from them.
Any unused product or waste material should be disposed of in accordance with local requirements.
If you use more Zomacton than you should:
An overdose may cause a decrease in blood sugar (hypoglycemia) and subsequently an increase in blood sugar (hyperglycemia).
If you or someone else has used more Zomacton than you should, contact your doctor or nearest hospital immediately. The effects of repeated overdose are unknown. Consult your doctor, pharmacist, or call the Toxicology Information Service, phone 91.562.04.20, indicating the medicine and the amount taken. It is recommended to take the packaging and the leaflet of the medicine to the healthcare professional.
If you forget to use Zomacton
If you forget a dose, do not worry. Continue normally and administer the next dose at the usual time.
Do not use a double dose to make up for the forgotten dose.
You may experience hypoglycemia (low blood sugar levels) that can cause dizziness, confusion, and blurred vision. Although the long-term effectiveness of the treatment will not be affected, you should consult your doctor if this happens to you.
4. Possible Adverse Effects
Like all medicines, Zomacton can cause adverse effects, although not all people suffer from them.
Subcutaneous injection of growth hormone may cause weight gain or loss, as well as occasional bleeding and hematoma (purple skin discoloration) at the injection site. It is therefore recommended to frequently change the injection site. In rare cases, patients developed skin pain or itching at the injection site.
Very common adverse effects,affecting more than 1 in 10 treated patients: Only adults:
- Swelling due to fluid retention, especially in hands and feet (Edema)
- Slightly elevated blood sugar levels (Hyperglycemia)
- Joint pain (Arthralgia)
- Muscle pain (Myalgia)
- Headache
- Numbness, tingling, burning, or prickling sensation on the skin (Paresthesia)
Common adverse effects,affecting between 1 and 10 out of 100 treated patients: Children and adults:
- Hypothyroidism
- Immune reaction to growth hormone, which may appear in a blood test (antibody formation)
- Headache
- Increased muscle tone stiffness (Hypertonia)
Only children:
- Swelling due to fluid retention, especially in hands and feet (Edema, peripheral edema)
- Injection site reactions
- Weakness (Asthenia)
- Modified glucose tolerance
- Joint pain (Arthralgia)
- Muscle pain (Myalgia)
Only adults:
- Stiffness in legs and/or arms
- Difficulty falling asleep and/or difficulty staying asleep (Insomnia)
Uncommon adverse effects, affecting between 1 and 10 out of 1,000 treated patients:
Children and adults:
- Anemia
- High heart rate (Tachycardia)
- Dizziness (Vertigo)
- Double vision (Diplopia)
- Papilla edema
- Vomiting, abdominal pain, flatulence, nausea
- Weakness
- Atrophy at the injection site, bleeding at the injection site, lump at the injection site, hypertrophy
- Low blood sugar levels (Hypoglycemia)
- Hyperphosphatemia (elevated phosphorus levels in blood)
- Muscle atrophy
- Bone pain
- Carpal tunnel syndrome
- Malignant neoplasia, neoplasia
- Somnolence
- Involuntary eye movements (Nystagmus)
- Personality disorders
- Urinary incontinence, hematuria (blood in urine), polyuria (increased urine volume), increased urination frequency, urinary anomalies
- Injection site reactions (including lipodystrophy, skin atrophy, exfoliative dermatitis, urticaria, hirsutism, skin hypertrophy)
- Increased breast size (Gynecomastia)
Only children:
- Stiffness in legs and/or arms
Only adults:
- High blood pressure (Hypertension)
Rare adverse effects, affecting between 1 and 10 out of 10,000 treated patients:
Children and adults:
- Diarrhea
- Abnormal kidney function tests
- Type 2 diabetes mellitus
- Numbness or tingling in certain areas of the body (Neuropathy)
- Fluid retention around the brain (manifested as repeated or intense headache, blurred vision, and nausea and/or vomiting)
Only children:
- High blood pressure (Hypertension)
- Difficulty falling asleep and/or difficulty staying asleep (Insomnia)
- Numbness, tingling, burning, or prickling sensation on the skin (Paresthesia)
Very rare adverse effects, affecting less than 1 in 10,000 treated patients:
Only children:
- Leukemia (the occurrence seems to be no more frequent than in children in the general population)
Reporting of Adverse Effects:
If you experience any adverse effects, consult your doctor or pharmacist, even if they are possible adverse effects not listed in this prospectus. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: http://www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Zomacton
Keep this medicine out of sight and reach of children.
Do not use Zomacton after the expiration date indicated on the packaging (CAD). The expiration date is the last day of the month indicated.
Store in a refrigerator at 2°C - 8°C; keep in the original packaging to protect from light.
Once the powder is dissolved in the provided solvent (reconstituted), store the vial in an upright position at 2°C and 8°C (in the refrigerator).
After reconstitution, the solution must be used within 28 days. Any remaining solution in the vial should be discarded at the end of this period.
In case of turbidity after refrigeration, wait until the solution reaches room temperature. If turbidity persists or discoloration appears, discard the vial and its contents.
Medicines should not be thrown away through wastewater or household waste. Deposit the packaging and unused medicines in the SIGRE collection point at the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and unused medicines. This will help protect the environment.
6. PACKAGE CONTENTS AND ADDITIONAL INFORMATION
Zomacton Composition
The active ingredient is somatropin 10 mg (10 mg/ml after reconstitution).
The other components are:
Powder: Mannitol, disodium phosphate dodecahydrate, sodium dihydrogen phosphate dihydrate.
Solvent: Water for injection and metacresol
What Zomacton looks like and package contents.
The product is a powder and solvent for injectable solution.
The powder is provided in a vial and the solvent in a syringe. The powder is white to off-white. When dissolved in the provided solvent, a clear and transparent solution is formed.
Zomacton is available in packs of 1, 3, and 5, and contains:
10 mg of somatropin in a vial and 1 ml of solvent in a syringe.
Not all packs are marketed.
Marketing Authorization Holder:
FERRING S.A.U.
C/ del Arquitecto Sánchez Arcas nº3, 1º
28040 Madrid
Spain
Manufacturer:FERRING GmbH
Wittland 11, D-24109 Kiel
GERMANY
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) with the following names:
Austria ZOMACTON 10 mg/ml – Powder and solvent for solution for injection
Belgium ZOMACTON 10 mg/ml
Denmark ZOMACTON 10 mg
Finland ZOMACTON 10 mg/ml
France Zomacton 10 mg/ml
Germany ZOMACTON 10 mg/ml
Greece ZOMACTON 10 mg
Ireland ZOMACTON 10 mg
Italy ZOMACTON 10 mg
Luxembourg ZOMACTON 10 mg/ml
Netherlands ZOMACTON 10 mg/ml
Portugal ZOMACTON 10 mg
Spain ZOMACTON 10 mg
Sweden ZOMACTON 10 mg/ml
United Kingdom (Northern Ireland) ZOMACTON 10 mg
This text was approved in September 2021.
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ZOMACTON 10 mg/ml POWDER AND SOLVENT FOR INJECTIONDosage form: INJECTABLE, 12 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription requiredDosage form: INJECTABLE, 5.3 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription requiredDosage form: INJECTABLE, 0.2 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription required
Alternatives to ZOMACTON 10 mg/ml POWDER AND SOLVENT FOR INJECTION in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to ZOMACTON 10 mg/ml POWDER AND SOLVENT FOR INJECTION in Poland
Alternative to ZOMACTON 10 mg/ml POWDER AND SOLVENT FOR INJECTION in Ukraine
Online doctors for ZOMACTON 10 mg/ml POWDER AND SOLVENT FOR INJECTION
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for ZOMACTON 10 mg/ml POWDER AND SOLVENT FOR INJECTION – subject to medical assessment and local rules.










