
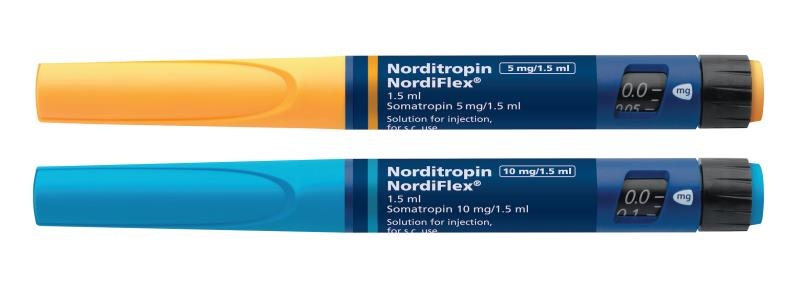
Norditropin Nordiflex

Ask a doctor about a prescription for Norditropin Nordiflex

How to use Norditropin Nordiflex
PATIENT INFORMATION LEAFLET
Information leaflet for the user: Norditropin NordiFlex 5 mg/1.5 ml, solution for injection in a pre-filled pen
Norditropin NordiFlex, 5 mg/1.5 ml, solution for injection in a pre-filled pen
Somatropin
Read this leaflet carefully before using this medicine, as it contains important information for you.
- You should keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
1. What Norditropin NordiFlex is and what it is used for
2. Before you use Norditropin NordiFlex
3. How to use Norditropin NordiFlex
4. Possible side effects
5. How to store Norditropin NordiFlex
6. Contents of the pack and other information
On the back:Instructions for using the Norditropin NordiFlex pen
1. What Norditropin NordiFlex is and what it is used for
Norditropin NordiFlex contains a biosynthetic human growth hormone called somatropin, which is identical to the growth hormone produced in the human body. Growth hormone is essential for normal growth in children and for normal body function in adults.
Norditropin NordiFlex is used to treat growth disorders in children:
- when the body does not produce enough growth hormone (growth hormone deficiency);
- Turner syndrome (a genetic disorder that may affect growth);
- kidney disease;
- low birth weight and born small for gestational age;
- Noonan syndrome (a genetic disorder that may impair growth).
Norditropin NordiFlex is used as replacement therapy in adults:
In adults, Norditropin NordiFlex is used to replace growth hormone when the body does not produce enough growth hormone due to a deficiency that started in childhood or developed during adulthood due to a tumor, treatment of a tumor, or a disease affecting the pituitary gland. In patients who were treated for growth hormone deficiency during childhood, a re-evaluation of the condition is necessary after completion of growth to determine if treatment should be continued.
2. Before you use Norditropin NordiFlex
When not to use Norditropin NordiFlex
- if you are allergic to somatropin, phenol, or any of the other ingredients of this medicine (listed in section 6);
- after a kidney transplant;
- if you have an active tumor (cancer). Tumors must be inactive and anti-tumor treatment must be completed before starting Norditropin NordiFlex;
- if you have a critical medical condition, such as open heart surgery, abdominal surgery, multiple injuries from an accident, or acute respiratory failure;
- after completion of growth (epiphyseal closure), if you do not have growth hormone deficiency.
Warnings and precautions
Before starting treatment with Norditropin NordiFlex, you should discuss the following with your doctor or pharmacist:
- if you have diabetes;
- if you have ever had cancer or any other type of tumor;
- if you experience recurring headaches, vision disturbances, nausea, or vomiting;
- if you have thyroid function disorders;
- in children, rapid growth may lead to an increase in curvature of the spine (scoliosis). During treatment with Norditropin NordiFlex, your doctor will check for signs of scoliosis in you (adult or child);
- if you limp or start limping during growth hormone treatment, you should inform your doctor;
- if you are over 60 years old or have used somatropin in adulthood for more than 5 years, due to limited experience;
- if you have kidney disease, your kidney function should be monitored by your doctor;
- if you are taking glucocorticoid replacement therapy, you should regularly consult your doctor, as your glucocorticoid dose may need to be adjusted;
- Norditropin NordiFlex may cause pancreatitis, which can cause severe abdominal and back pain. If you experience abdominal pain after taking Norditropin NordiFlex, you should contact your doctor.
Norditropin NordiFlex and other medicines
Tell your doctor or pharmacist about all the medicines you are taking, have recently taken, or might take, including:
- glucocorticoids - concurrent use of Norditropin NordiFlex and glucocorticoids may affect growth in adults;
- cyclosporine (an immunosuppressive drug) - dose adjustment may be necessary;
- insulin - dose adjustment may be necessary;
- thyroid hormone - dose adjustment may be necessary;
- gonadotropin (a hormone that stimulates the sex glands) - dose adjustment may be necessary;
- antiepileptic drugs - dose adjustment may be necessary;
- orally administered estrogens or other sex hormones.
Pregnancy and breastfeeding
Somatropin-containing medicines are not recommended for use in women of childbearing potential who are not using contraception.
- Pregnancy- If you become pregnant while using Norditropin NordiFlex, you should stop treatment and consult your doctor.
- Breastfeeding- Do not use Norditropin NordiFlex during breastfeeding, as somatropin may pass into breast milk.
Driving and using machines
Norditropin NordiFlex does not affect the ability to drive and use machines.
Norditropin contains sodium
Norditropin contains less than 1 mmol of sodium (23 mg) per 1.5 ml, which is essentially "sodium-free".
3. How to use Norditropin NordiFlex
This medicine should always be used as directed by your doctor. If you are unsure, consult your doctor or pharmacist.
Recommended dose
The recommended dose for children depends on body weight and body surface area. The recommended dose for adults depends on height, body weight, sex, and sensitivity to growth hormone; it should be adjusted until the required dose is achieved.
- Children with insufficient growth hormone secretion or growth hormone deficiency:The usual recommended dose is 0.025 to 0.035 mg per kg body weight per day or 0.7 to 1.0 mg per square meter body surface area per day.
- Children with Turner syndrome:The usual recommended dose is 0.045 to 0.067 mg per kg body weight per day or 1.3 to 2.0 mg per square meter body surface area per day.
- Children with kidney disease:The usual recommended dose is 0.050 mg per kg body weight per day or 1.4 mg per square meter body surface area per day.
- Children born small for gestational age:The usual recommended dose is 0.035 mg per kg body weight per day or 1.0 mg per square meter body surface area per day until the target height is reached. (In clinical trials in children born small for gestational age, doses of 0.033 and 0.067 mg per kg body weight per day were usually used).
- Children with Noonan syndrome:The usual recommended dose is 0.066 mg per kg body weight per day, but your doctor may decide that a dose of 0.033 mg per kg body weight per day is sufficient.
- Adults with insufficient growth hormone secretion or growth hormone deficiency:If growth hormone deficiency persists after completion of growth, treatment should be continued. The usual initial dose is 0.2 to 0.5 mg per day. The dose is adjusted over time to establish the appropriate dose. In adults with growth hormone deficiency, the usual initial dose is 0.1 to 0.3 mg per day. This dose is increased at monthly intervals until the appropriate dose for the individual patient is reached. The maximum daily dose is usually 1.0 mg.
When to use Norditropin NordiFlex
The recommended daily dose should be injected subcutaneously every evening, just before bedtime.
How to use Norditropin NordiFlex
Norditropin NordiFlex contains a solution of growth hormone in a pre-filled, single-use, 1.5 ml pen.
- Check the solution before use by turning the pen upside down once or twice. Do not use the pen if the solution is cloudy or has changed color.
- Norditropin NordiFlex is intended for use with NovoFine or NovoTwist single-use needles up to 8 mm in length.
- Always use a new needle for each injection.
- Change the injection site to avoid damaging the skin.
- To ensure delivery of the full dose and to avoid injecting air, always check the flow before performing the first injection with a new Norditropin NordiFlex pen.
Never share your Norditropin NordiFlex pen with others.
- Do not use Norditropin NordiFlex if you have any other growth hormone deficiency that is not specified in this leaflet.
How long to use Norditropin NordiFlex
- Children with growth disorders due to Turner syndrome, kidney disease, born small for gestational age, or Noonan syndrome: treatment should be continued until the end of growth, as directed by your doctor.
- Children or adolescents with growth hormone deficiency: treatment should be continued until adulthood, as directed by your doctor.
Do not stop using Norditropin NordiFlex without first discussing it with your doctor.
Using more Norditropin NordiFlex than prescribed
If you have taken more somatropin than prescribed, contact your doctor. Long-term overdose may cause excessive growth and coarsening of facial features.
Missing a dose of Norditropin NordiFlex
Take the next dose at the usual time. Do not take a double doseto make up for a missed dose.
Stopping Norditropin NordiFlex treatment
Do not stop using Norditropin NordiFlex without first discussing it with your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Side effects seen in children and adults(frequency not known):
- rash, wheezing, swelling of the eyelids, face, or lips, loss of consciousness. These may be symptoms of an allergic reaction;
- headache, vision problems, nausea, and vomiting. These may be symptoms of increased intracranial pressure;
- thyroxine levels in the blood may be decreased;
- hyperglycemia(elevated blood sugar levels). If you experience any of these side effects, contact your doctor immediately. You should stop using Norditropin NordiFlex until your doctor advises you to continue treatment.
Rarely, the formation of antibodies against somatropin has been observed during Norditropin treatment.
Elevated liver enzyme activity has been reported.
In patients treated with somatropin (the active substance in Norditropin NordiFlex), cases of leukemia and brain tumor recurrence have been reported, but there is no evidence of a link between somatropin and their occurrence.
If you suspect you have any of these conditions, you should discuss it with your doctor.
Additional side effects in children:
Uncommon(may affect up to 1 in 100 children):
- headache,
- redness, itching, and pain at the injection site,
- breast enlargement(gynecomastia).
Rare(may affect up to 1 in 1,000 children):
- rash,
- muscle and joint pain,
- swelling of the handsand feet due to fluid retention in the body. In rare cases, children receiving Norditropin NordiFlex may experience knee or hip pain or start limping. These symptoms may be caused by a condition affecting the upper end of the thigh bone (Legg-Calvé-Perthes disease) or slipping of the hip joint (slipped capital femoral epiphysis), rather than the use of Norditropin NordiFlex.
In clinical trials in children with Turner syndrome, a few cases of excessive growth of the hands and feet in relation to body height have been observed.
A clinical trial in children with Turner syndrome showed that high doses of Norditropin may increase the risk of ear infections.
If any of the side effects get worse or if you notice any side effects not listed in this leaflet, you should tell your doctor or pharmacist.
Additional side effects in adults:
Very common(may affect more than 1 in 10 adults):
- swelling of the handsand feet due to fluid retention in the body.
Common(may affect up to 1 in 10 adults):
- headache,
- tingling sensation, numbness, and pain, mainly in the fingers,
- joint painand stiffness, muscle pain.
Uncommon(may affect up to 1 in 100 adults):
- type 2 diabetes,
- carpal tunnel syndrome, tingling, and pain in the fingers and hands,
- itching(may be intense) and pain at the injection site,
- muscle stiffness,
- breast enlargement(gynecomastia).
Reporting side effects
If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly to the national reporting system via the contact details listed below. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Norditropin NordiFlex
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label after "EXP". The expiry date refers to the last day of that month.
Before use, store the Norditropin NordiFlex pen in a refrigerator (2°C – 8°C) in the outer packaging to protect it from light. Do not freeze or expose to high temperatures. Do not store near any cooling element.
While usingNorditropin NordiFlex 5 mg/1.5 ml, you can:
- store it in a refrigerator (2°C - 8°C) for up to 4 weeks or
- store it at room temperature (below 25°C) for up to 3 weeks.
Do not use Norditropin NordiFlex pens that have been frozen or exposed to high temperatures.
Do not use the Norditropin NordiFlex pen if the growth hormone solution in the cartridge is cloudy or has changed color.
Always store the Norditropin NordiFlex pen without an attached needle.
Always put the pen cap back on the Norditropin NordiFlex pen when not in use.
Always use a new needle for each injection.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
What Norditropin NordiFlex contains
- The active substance is somatropin.
- The other ingredients are mannitol, histidine, poloxamer 188, phenol, water for injections, hydrochloric acid (for pH adjustment), and sodium hydroxide (for pH adjustment).
What Norditropin NordiFlex looks like and contents of the pack
Norditropin NordiFlex is a clear and colorless solution for injection in a pre-filled, single-use, 1.5 ml pen.
1 ml of solution contains 3.3 mg of somatropin.
1 mg of somatropin corresponds to 3 IU of somatropin.
Norditropin NordiFlex is available in strengths of:
5 mg/1.5 ml (corresponding to 3.3 mg/ml),
10 mg/1.5 ml (corresponding to 6.7 mg/ml).
Pack sizes: 1 pre-filled pen, and packs of 5 and 10 pre-filled pens. Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd
Denmark
This medicine is authorized in the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Austria, Belgium, Croatia, Cyprus, Czech Republic, Denmark, Finland, Greece, Spain, Ireland, Iceland, Lithuania, Luxembourg, Norway, Poland, Portugal, Romania, Slovakia, Sweden, Hungary, United Kingdom (Northern Ireland), Italy: Norditropin NordiFlex 5 mg/1.5 ml
France: Norditropine NordiFlex 5 mg/1.5 ml
Date of last revision of the leaflet11/2022
Further information
Detailed information on this medicine is available on the website of the Medicines Agency.
Norditropin NordiFlex
5 mg/1.5 ml
Instructions for using the Norditropin NordiFlex pen
Read these instructions carefully before using Norditropin NordiFlex.
- Norditropin NordiFlex 5 mg/1.5 ml is a pre-filled, single-use, 1.5 ml pen containing a solution of human growth hormone.
- The dose can be set in increments of 0.025 mg using the dose selector. The dose prescribed by your doctor should be set.
- Norditropin NordiFlex is intended for use with NovoFine or NovoTwist single-use needles up to 8 mm in length.
- Always start by checking the name, strength, and color of the Norditropin NordiFlex pen to ensure it contains the correct strength of growth hormone for you.
- Use the pen only if the growth hormone solution in the cartridge is clear and colorless.
- Always use a new needle for each injection.
- Always check the flow before performing the first injection with a new Norditropin NordiFlex pen - see section 3. Checking the flow.
- Never share your Norditropin NordiFlex pen or needles with others. This can lead to cross-infection.
- Always store the pen and needles out of the sight and reach of children.
- Caregivers should exercise extreme caution when removing and disposing of used needles to minimize the risk of needlestick injury and cross-infection.
| Needle (example) Pen cap Outer needle guard Inner needle guard Needle Cartridge Paper label Pen window Dose scale Dose button Dose counter | |
| A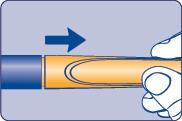 |
| B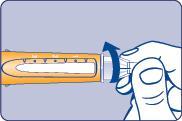 |
|  |
| C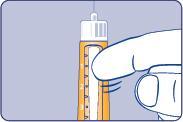 |
| D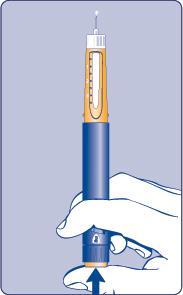 |
| E |
| F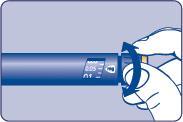 |
| G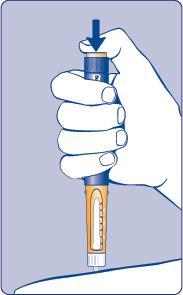 |
| H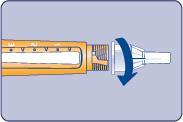 |
| |
- For information on storing the pen, see section 5. How to store Norditropin NordiFlex on the back of this leaflet.
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterNovo Nordisk A/S
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Norditropin NordiflexDosage form: Powder, 12 mg (36 IU)Active substance: somatropinManufacturer: Pfizer Manufacturing Belgium NVPrescription requiredDosage form: Powder, 5.3 mg (16 IU)Active substance: somatropinManufacturer: Pfizer Health AB Pfizer Manufacturing Belgium NVPrescription requiredDosage form: Solution, 10 mg/1.5 mlActive substance: somatropinPrescription required
Alternatives to Norditropin Nordiflex in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Norditropin Nordiflex in Spain
Alternative to Norditropin Nordiflex in Ukraine
Online doctors for Norditropin Nordiflex
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Norditropin Nordiflex – subject to medical assessment and local rules.










