

Genotropin 5,3

Ask a doctor about a prescription for Genotropin 5,3

How to use Genotropin 5,3
Package Leaflet: Information for the Patient
GENOTROPIN 5.3, 5.3 mg (16 IU), powder and solvent for solution for injection
GENOTROPIN 12, 12 mg (36 IU), powder and solvent for solution for injection
Somatropin
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. See section 4.
Table of Contents
- 1. What is Genotropin and what is it used for
- 2. Important information before using Genotropin
- 3. How to use Genotropin
- 4. Possible side effects
- 5. How to store Genotropin
- 6. Contents of the pack and other information
1. What is Genotropin and what is it used for
Genotropin contains somatropin, a human growth hormone produced in Escherichia colibacteria using recombinant DNA technology. Somatropin is necessary for normal growth in children. In adults with growth hormone deficiency, somatropin reduces fat mass and increases muscle mass.
Genotropin is used in children:
- with growth disorders caused by insufficient growth hormone secretion;
- with growth disorders associated with Turner syndrome (abnormal chromosome structure in girls);
- with growth disorders associated with chronic renal failure;
- with growth disorders in low-birth-weight children who were born with a weight too low for their gestational age;
- with Prader-Willi syndrome, to improve growth and body composition.
Genotropin is used in adults as replacement therapy for those with significant growth hormone deficiency. Treatment can be started in adulthood or continued from childhood.
If the patient was treated with Genotropin in childhood, after the growth is complete, the growth hormone levels should be checked. If a severe growth hormone deficiency is found, the doctor may recommend continuing treatment.
2. Important information before using Genotropin
Before starting treatment with Genotropin, the diagnosis must be confirmed. Treatment should only be carried out under the supervision of a doctor with the necessary knowledge and experience.
When not to use Genotropin
- if the patient is allergic to somatropin or any of the other ingredients of this medicine (listed in section 6);
- if the patient has an active tumor disease. The doctor should be informed about the tumor disease. Tumors must be inactive, and anti-tumor therapy must be completed before starting treatment with Genotropin;
- in children who have completed growth;
- if the patient has life-threatening acute illnesses, complications after open-heart surgery, abdominal surgery, and multiple organ injuries, or acute respiratory failure, etc. Before planned surgery or during hospitalization, the doctor should be informed about the use of growth hormone.
Warnings and precautions
Before starting treatment with Genotropin, the following should be discussed with the doctor or pharmacist:
- in patients with diabetes; after starting Genotropin, it may be necessary to adjust the dose of the diabetes medication;
- if there are risk factors for diabetes (e.g., if the patient is overweight or has a family history of diabetes);
- in some patients, after starting treatment with Genotropin, it may be necessary to start replacement therapy with thyroid hormones;
- in patients treated with thyroid hormones, it may be necessary to change the dosage of these medications;
- if the patient is using replacement therapy with glucocorticoids, they should regularly consult with their doctor, as it may be necessary to adjust the dose of the medication;
- if the patient starts to limp (only applies to children);
- in case of severe or sudden headaches, vision disturbances, nausea, and/or vomiting;
- when symptoms of scoliosis occur;
- if the patient experiences increasing abdominal pain, they should inform their doctor;
- in people over 80 years old, due to limited experience in treating this age group and a higher risk of side effects.
If treatment is related to chronic renal impairment, kidney function should be evaluated before starting treatment; after kidney transplantation, Genotropin should be discontinued.
In Prader-Willi syndrome, before starting treatment and during its duration, the doctor should pay attention to the following risk factors: severe obesity, respiratory disorders, or sleep apnea in history, as well as unidentified respiratory infections.
The doctor will recommend a low-calorie diet and/or treat the infection.
In patients with intrauterine growth retardation, other causes of growth disorders should be excluded before starting treatment. The doctor will order a test to determine insulin and glucose levels before starting treatment, and then once a year, as well as determine the level of IGF-I (insulin-like growth factor) before starting treatment and then twice a year. It is not recommended to start therapy in children with intrauterine growth retardation who are approaching puberty.
Genotropin and other medicines
Tell your doctor about all the medicines you are taking now or have taken recently, as well as any medicines you plan to take.
In particular, inform your doctor if you are taking or have recently taken the following medicines:
- orally administered estrogens or other sex hormones. The doctor may recommend adjusting the dose of Genotropin or other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Genotropin should not be used in pregnant or breastfeeding women or in women who plan to become pregnant in the near future.
Driving and using machines
Genotropin has no influence on the ability to drive and use machines.
Genotropin contains m-cresol
Genotropin contains m-cresol as an excipient, which may be associated with muscle inflammation. You should report muscle pain or excessive pain at the injection site to your doctor. If muscle inflammation is confirmed, your doctor will decide on the use of a medicine that does not contain m-cresol.
Genotropin contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per maximum daily dose, which means it is essentially 'sodium-free'.
3. How to use Genotropin
This medicine should always be used as directed by your doctor. If you are unsure, consult your doctor or pharmacist.
The doctor will determine the dose individually for each patient.
Genotropin is administered subcutaneously. The injection site should be changed to avoid skin changes.
Follow the doctor's or nurse's instructions.
Growth disorders in children caused by insufficient growth hormone secretion
The recommended dose is: 0.025-0.035 mg/kg body weight per day or 0.7-1.0 mg/m2 body surface area per day.
In children with Prader-Willi syndrome, to accelerate growth and improve body composition
The recommended dose is: 0.035 mg/kg body weight per day or 1.0 mg/m2 body surface area per day.
Do not use a daily dose greater than 2.7 mg/m2 body surface area per day. Do not treat children who grow less than 1 cm per year and will soon complete growth.
Growth disorders in children associated with Turner syndrome
The recommended dose is: 0.045-0.050 mg/kg body weight per day or 1.4 mg/m2 body surface area per day.
Growth disorders associated with chronic renal failure
The recommended dose is: 0.045-0.050 mg/kg body weight per day or 1.4 mg/m2 body surface area per day. If the growth rate is too slow, it may be necessary to increase the dose. Dose adjustment may be necessary after 6 months of treatment.
Growth disorders in low-birth-weight children who were born with a weight too low for their gestational age
The recommended dose is: 0.035 mg/kg body weight per day (1 mg/m2 body surface area per day). Treatment continues until the final height is reached. Treatment should be stopped if the growth rate is too slow.
Adults with growth hormone deficiency
In patients who continue growth hormone therapy after childhood GH deficiency, the recommended dose to resume treatment is 0.2-0.5 mg per day. The dose should be gradually increased or decreased according to the individual needs of the patient, based on the level of IGF-I (insulin-like growth factor).
In patients with adult-onset GH deficiency, treatment should be started with a low dose: 0.15-0.3 mg per day. The dose is increased gradually, depending on the level of IGF-I (insulin-like growth factor) in the serum, as well as the efficacy and side effects of the treatment.
The maximum daily dose rarely exceeds 1.0 mg per day. The smallest effective dose should be used.
Women may require higher doses than men.
Natural hormone production decreases with age, so it may be necessary to reduce the dose over time.
Dosing should be monitored every 6 months.
In patients over 60 years old, treatment should be started with a dose of 0.1-0.2 mg per day and gradually increased according to the individual needs of the patient. The smallest effective dose should be used. The maintenance dose in these patients rarely exceeds 0.5 mg per day.
Method of administration
Genotropin solution for injection in a GoQuick cartridge. The cartridge, which is pre-assembled in the injector, contains the growth hormone and solvent.
Remember to screw the needle onto the GoQuick injector before mixing. Use a new needle for each injection. The needle must not be reused.
Mixing the growth hormone and solvent is done by screwing the cartridge into the injector (see GoQuick User Manual). Then, dissolve the powder by gently tilting the injector until the powder is completely dissolved.
DO NOT SHAKE!
Shaking can cause denaturation of the active substance, leading to loss of biological properties.
Read the instructions for use of the GoQuick injector, which is included in the package leaflet for the patient.
Using more Genotropin than recommended
If you have injected too much Genotropin, contact your doctor. A single overdose can affect blood glucose levels, which may become too high or too low. You may experience shakiness, sweating, sleepiness, fainting, or a feeling of being unwell.
Missing a dose of Genotropin
Do not use a double dose to make up for a missed dose.
If you have any further questions about using this medicine, ask your doctor.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common (may affect more than 1 in 10 people):
Adults:
- Joint pain.
- Fluid retention (swollen fingers or wrists).
Common (may affect up to 1 in 10 people):
Children:
- Joint pain.
- Temporary redness, itching, and pain at the injection site.
Adults:
- Numbness/tingling.
- Pain or burning sensation in the hands or feet (known as carpal tunnel syndrome).
- Stiffness of the arms and legs, muscle pain.
Uncommon (may affect up to 1 in 100 people):
Children:
- Leukemia (occurred in a small number of patients with growth hormone deficiency, some of whom were treated with Genotropin. However, there is no evidence that the occurrence of leukemia is increased in patients with growth hormone deficiency without predisposing factors).
- Increased intracranial pressure (which can cause symptoms such as severe headache, vision disturbances, or vomiting).
- Numbness/tingling.
- Rash.
- Itching.
- Raised, itchy bumps on the skin.
- Muscle pain.
- Breast enlargement (gynecomastia).
- Fluid retention (swollen fingers or wrists).
Adults:
- Breast enlargement (gynecomastia).
Frequency not known (cannot be estimated from the available data):
Adults and children:
- Type 2 diabetes.
- Facial swelling.
- Headache.
- Decreased cortisol levels in the blood.
Children:
- Stiffness of the arms and legs.
Adults:
- Increased intracranial pressure (which can cause symptoms such as severe headache, vision disturbances, or vomiting).
- Rash.
- Itching.
- Raised, itchy bumps on the skin.
- Redness, itching, and pain at the injection site.
Antibodies against the administered growth hormone have been observed, but this does not appear to be related to a cessation of its effect.
Very rarely, inflammatory reactions of the muscles at the injection site, caused by m-cresol (an excipient), have been observed. If the doctor confirms the occurrence of such a reaction, it is recommended to use a medicine that does not contain m-cresol.
The injection site should be changed to avoid skin changes in the form of unevenness or lumps.
After the marketing of the medicine, there have been reports of rare cases of sudden death in patients with Prader-Willi syndrome who were treated with Genotropin. However, a causal relationship with Genotropin has not been established.
In children treated with growth hormone, cases of slipped capital femoral epiphysis and Legg-Calve-Perthes disease have been reported. However, a causal relationship with Genotropin has not been established.
Other side effects associated with the use of growth hormone may include increased blood sugar levels or decreased thyroid hormone levels in adults and children. This can be confirmed by the doctor, and if necessary, they may initiate appropriate treatment. Rarely, cases of pancreatitis have been reported in patients treated with growth hormone.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, please tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety, Ministry of Health
Al. Jerozolimskie 181C
02-222 Warsaw
Tel.: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder or its representative.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Genotropin
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton after 'EXP'. The expiry date refers to the last day of that month.
Store in a refrigerator (2°C - 8°C). Do not freeze. The medicine can be stored at a temperature below 25°C for one month. Keep the outer carton in order to protect from light.
After reconstitution
The solution can be stored for 28 days in a refrigerator (2°C - 8°C). Do not freeze. Keep the outer carton in order to protect from light.
If a protective needle cap is used, the injector should be stored with the protective needle cap and the black plug.
If a protective needle cap is not used, the injector should be stored with the white plug (see User Manual).
These actions will help protect the medicine from light.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and other information
What Genotropin 5.3 contains
- The active substance is somatropin*. One cartridge contains 5.3 mg of somatropin*.
- The other ingredients are: glycine, mannitol, sodium dihydrogen phosphate monohydrate (anhydrous), sodium phosphate dodecahydrate (anhydrous) (see section 2 "Genotropin contains sodium"). The solvent contains m-cresol (see section 2 "Genotropin contains m-cresol"), mannitol, water for injections. After reconstitution, the somatropin concentration is 5.3 mg/ml.
* produced in Escherichia colibacteria using recombinant DNA technology.
What Genotropin 12 contains
- The active substance is somatropin*. One cartridge contains 12 mg of somatropin*.
- The other ingredients are: glycine, mannitol, sodium dihydrogen phosphate monohydrate (anhydrous), sodium phosphate dodecahydrate (anhydrous) (see section 2 "Genotropin contains sodium"). The solvent contains m-cresol (see section 2 "Genotropin contains m-cresol"), mannitol, water for injections. After reconstitution, the somatropin concentration is 12 mg/ml.
* produced in Escherichia colibacteria using recombinant DNA technology.
What Genotropin looks like and contents of the pack
Genotropin is available in cartridges, one chamber of which contains the active substance as a white powder, and the other chamber contains a clear solvent for the active substance.
Genotropin is available in packs containing 1 or 5 cartridges.
Marketing authorization holder:
Pfizer Europe MA EEIG
Boulevard de la Plaine 17
1050 Brussels
Belgium
Manufacturer:
Pfizer Manufacturing Belgium NV
Rijksweg 12
2870 Puurs
Belgium
To obtain more detailed information on this medicine, please contact the representative of the marketing authorization holder:
Pfizer Polska Sp. z o.o.
tel.: 22 335 61 00
Date of last revision of the leaflet: 05/2024
Other sources of information
Detailed information on this medicine is available on the website of the Ministry of Health http://urpl.gov.pl
What Genotropin 12 contains
- The active substance of the medicine is somatropin*. One cartridge contains 12 mg of somatropin*.
- The other ingredients are: glycine, mannitol, sodium dihydrogen phosphate monohydrate (calculated as anhydrous), sodium hydrogen phosphate dodecahydrate (calculated as anhydrous) (see section 2 "Genotropin contains sodium"). The solvent contains m-cresol (see section 2 "Genotropin contains m-cresol"), mannitol, water for injections. After reconstitution, the somatropin concentration is 12 mg/ml.
* produced in Escherichia colicells by DNA recombinant technology.
What Genotropin looks like and what the pack contains
Genotropin is available in pre-filled, multi-dose, single-use GoQuick syringes containing dual-chamber cartridges, where one chamber contains the active substance in the form of a white powder, and the other contains a clear solution that is the solvent for the active substance.
Genotropin is available in a pack containing one or five GoQuick syringes.
Responsible entity:
Pfizer Europe MA EEIG
Boulevard de la Plaine 17
1050 Brussels
Belgium
Manufacturer:
Pfizer Manufacturing Belgium NV
Rijksweg 12
2870 Puurs
Belgium
To obtain more detailed information about this medicine, please contact the representative of the responsible entity:
Pfizer Polska Sp. z o.o.
tel.: 22 335 61 00
Date of last update of the leaflet: 05/2024
Other sources of information
Detailed information about this medicine is available on the website of the Office for Registration of Medicinal Products, Medical Devices and Biocidal Products http://urpl.gov.pl
GENOTROPIN 5.3 GOQUICK INSTRUCTIONS FOR USE
Read the entire instructions before using the GoQuick syringe. If you have any questions about the recommended dose or treatment with Genotropin 5.3, please contact your doctor or nurse.
Information about the GoQuick syringe
GoQuick is a pre-filled, multi-dose, single-use syringe containing 5.3 mg of somatropin. It can be used to administer Genotropin 5.3 in doses ranging from 0.1 mg to 1.5 mg. Each click of the black ring changes the dose by 0.05 mg. The Genotropin 5.3 in the syringe is mixed only once, at the first use of the syringe. The cartridges in the syringe are not interchangeable. After the syringe is empty, a new one should be used.
The syringe has a dose memory. The dose is set once in the syringe. Then, with each injection, it is set to administer the same dose. This prevents exceeding the set dose.
Important information
- Do notmix the powder and liquid in the syringe if there is no needle on it.
- Do notstore the syringe with the needle attached. Genotropin 5.3 may leak out, and air bubbles may form in the cartridge. Before preparing for storage, always remove the needle and attach the syringe cap or the protective needle cap.
- Be careful not to drop the syringe. If it is dropped and any part of it appears to be broken or damaged, do notuse it. Contact your doctor or nurse to obtain a new syringe. If the syringe is dropped but not damaged or broken, perform the dose reloading procedure according to the instructions in step 6 (Setting up and using a new GoQuick syringe).
- Clean the syringe with a damp cloth. Do notimmerse it in water.
- Use a new needle for each injection. Do notshare needles for syringes.
- The remaining volume scale on the side of the cartridge holder indicates the volume of Genotropin 5.3 remaining in the syringe.
Storage and disposal
- Store the syringe in the refrigerator (at a temperature between 2°C and 8°C), in the outer packaging, to protect it from light. Do notfreeze or expose to frost.
- Do notuse the syringe after the expiration date.
- After 28 days from mixing, discard the syringe, even if some medicine remains.
- Information on storing the GoQuick syringe can be found in this leaflet, on its second page.
- Dispose of the used syringe according to local regulations. In case of doubt, consult a doctor or nurse.
Components of the GoQuick syringe
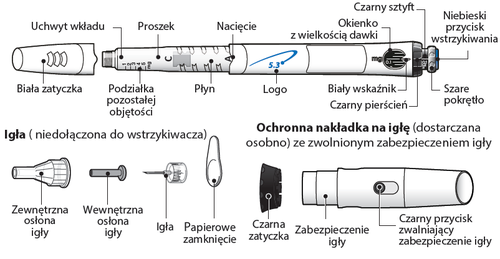
Needles are notincluded with the GoQuick syringe. Needles up to 8 mm in length should be purchased at a pharmacy.
- Needles for use with the GoQuick syringe: 31 G or 32 G (Becton Dickinson and Company) 31 G or 32 G (Novo Nordisk) 32.5 G or 34 G (Terumo)
Setting up and using a new GoQuick syringe
Step 1 Preparation

- Wash and dryyour hands.
- Gatherthe following materials and place them on a clean, flat surface: a new GoQuick syringe, a new needle (not included in the kit), and a suitable container for sharp waste (not included in the kit).
- Checkthe expiration date on the syringe label. Do notuse the syringe if the expiration date has passed.
Step 2 Choosing the injection site
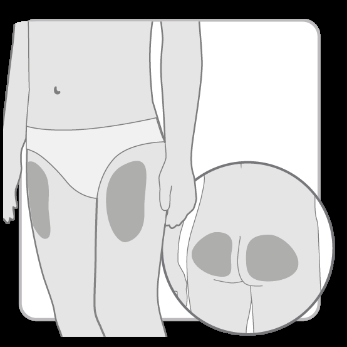
- Choose and cleanthe injection site according to the doctor's or nurse's recommendations. Choose a different site for each injection. Each subsequent injection should be performed at a site at least 2 cm away from the previous one.
- Avoid bony areas, bruised, red, painful, or hardened areas, as well as areas of skin covered with scars or affected by disease.
Step 3 Attaching a new needle

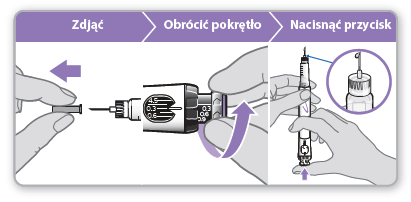
- Removethe white plug from the syringe.
- Take a new needle and remove the paper closure from it.
- Gently press and then screwthe needle onto the syringe. Do notovertighten. Caution:Be careful not to attach the needle at an angle. This may cause leakage.
- Leave both shields on the needle.
Step 4 Mixing Genotropin 5.3

- Holdthe syringe with the needle pointing upwards and the letter "A" towards you.
- Tightly screwthe cartridge into the syringe until you feel a click, when the letter "B" is in the notch.
- Gently tiltthe syringe from side to side to facilitate complete dissolution of the powder. Do notshake. Shaking may damage the growth hormone.
- Checkthat the liquid in the cartridge is clear and that all the powder has dissolved. o If the liquid is cloudy or powder is visible, gently tilt the syringe from side to side a few more times. o If the liquid is still cloudy or powder is visible, do notuse this syringe and try again with a new one.
Step 5 Removing air from the syringe

- Removethe outer needle shield. Keep it to secure the used needle after injection.
- Leave the inner needle shield in place. Caution:After removing the outer shield, the inner shield should be visible. If it is not visible, try to attach the needle again.
- Holdthe syringe with the needle pointing upwards.
- Gently tapthe cartridge to cause the trapped air to move upwards.
- Tightly screwthe cartridge into the syringe until you feel a click, when the letter "C" is in the notch. A small amount of liquid may appear around the inner needle shield. This is normal.
Step 6 Loading the dose
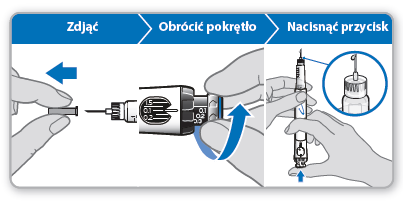
Loading the dose allows for the removal of any remaining air by expressing a small amount of liquid from the syringe. The loaded dose is 0.1 mg and differs from the dose prescribed by the doctor or nurse. This step should only be performed when using the syringe for the first time.
- Removeand discard the inner needle shield. Caution:Do not touch the needle to avoid pricking.
- Make surethat the dose of 0.1 mg is set in the dose window.
- Turnthe gray dial in the direction indicated by the arrows until it stops clicking.
- Holdthe syringe with the needle pointing straightupwards.
- Pressthe blue injection button until it stops clicking.
- Checkif there is liquid at the tip of the needle. If it appears, the syringe is ready for use. o If the liquid does not appear, repeat the steps described in the dose loading step, once or twice. o If the liquid still does not appear, do not use this syringe. Contact your doctor or nurse for advice.
Step 7 Setting and preparing the dose

When using the syringe for the first time, the dose prescribed by the doctor or nurse will be set.
There is no need to reset the dose until a new syringe is started or until the doctor or nurse advises to do so.
- Turn the black ring counterclockwiseuntil the recommended dose is aligned with the white pointer in the dose window. Be careful not to turn the gray dial.o If the selected dose is misaligned with the white pointer, turn the black ring in the other direction to set the correct dose value. Caution:If the black ring cannot be turned, press the blue injection button until it stops clicking. Then try to set the dose again. Remember that liquid will be expressed from the needle. Turn the gray dialin the direction indicated by the arrows until it stops clicking.
Step 8 Checking the dose
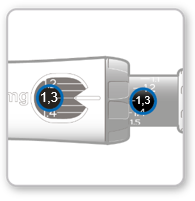
The selected dose value visible on the black rod should be alignedwith the white pointer.
- Make surethat the dose value visible on the black rod matches the value set in the dose window. o If these values are the same, the syringe is ready for injection. o If the dose values do not match, make sure the gray dial was turned in the correct direction indicated by the arrows until it stopped clicking.
Step 9 Injecting Genotropin 5.3
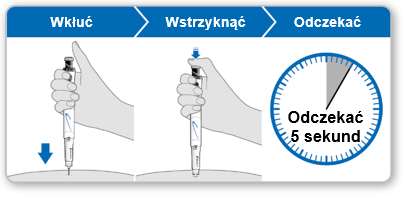
- Holdthe syringe over the injection site.
- Insertthe needle straight into the skin.
- Pressthe blue injection button until it stops clicking.
- Waitfor 5 full seconds to ensure the full dose is injected. During this time, maintain gentle pressure on the blue injection button.
- After 5 seconds, remove the needle from the skin without tilting it to the sides. Caution:If a drop of liquid is visible at the injection site or at the tip of the needle, during the next injection, hold the pressed blue injection button for a longer time before removing the needle from the skin.
Step 10 Removing the needle

- Carefully slidethe outer shield onto the needle. Caution:Do not touch the needle to avoid pricking.
- Unscrewthe needle using the outer shield.
- Discardthe needle into a suitable container for sharp waste.
- Slidethe white plug onto the syringe.
- Store the syringe in the refrigerator until the next injection.
Regular (daily) use of the GoQuick syringe
Step 1 Preparation

- Wash and dryyour hands.
- Gatherthe following materials and place them on a clean, flat surface: the GoQuick syringe with the mixed medicine, a new needle (not included in the kit), and a suitable container for sharp waste (not included in the kit).
- Checkthe expiration date on the syringe label. Do notuse the syringe if the expiration date has passed. Do notuse the syringe after 28 days from the first use.
Step 2 Choosing the injection site
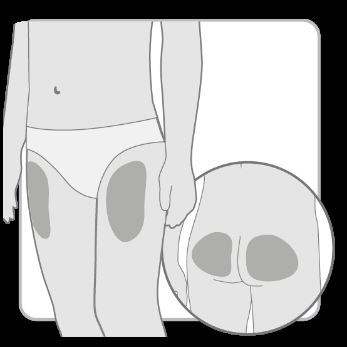
- Choose and cleanthe injection site according to the doctor's or nurse's recommendations. Choose a different site for each injection. Each subsequent injection should be performed at a site at least 2 cm away from the previous one.
- Avoid bony areas, bruised, red, painful, or hardened areas, as well as areas of skin covered with scars or affected by disease.
Step 3 Attaching a new needle

- Removethe white plug from the syringe.
- Take a new needle and remove the paper closure from it.
- Gently press and then screwthe needle onto the syringe. Do notovertighten. Caution:Be careful not to attach the needle at an angle. This may cause leakage.
- Removeboth shields from the needle. Keep the outer needle shield to secure the needle after injection.
Step 4 Loading the dose

- Turn the gray dialin the direction indicated by the arrows until it stops clicking.
- The selected dose value visible on the black rod should be alignedwith the white pointer.
- Make surethat the dose value visible on the black rod matches the value set in the dose window. o If these values are the same, the syringe is ready for injection. Caution:If the prepared dose is smaller, it means that the syringe does not contain a full dose of Genotropin 5.3. If the syringe does not contain a full dose, follow the doctor's or nurse's instructions or contact them for advice.
Step 5 Injecting Genotropin 5.3
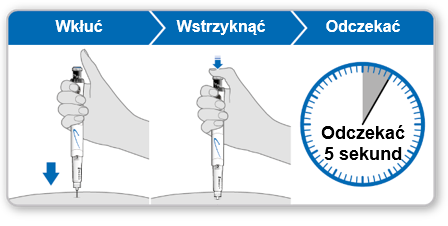
- Holdthe syringe over the injection site.
- Insertthe needle straight into the skin.
- Pressthe blue injection button until it stops clicking.
- Waitfor 5 full seconds to ensure the full dose is injected. During this time, maintain gentle pressure on the blue injection button.
- After 5 seconds, remove the needle from the skin without tilting it to the sides. Caution:If a drop of liquid is visible at the injection site or at the tip of the needle, during the next injection, hold the pressed blue injection button for a longer time before removing the needle from the skin.
Step 6 Removing the needle

- Carefully slidethe outer shield onto the needle. Caution:Do not touch the needle to avoid pricking.
- Unscrewthe needle using the outer shield.
- Discardthe needle into a suitable container for sharp waste.
- Slidethe white plug onto the syringe.
- Store the syringe in the refrigerator until the next injection.
Using the protective needle cap (optional)
The protective needle cap is an optional component provided separately, which allows you to cover the needle during injection.
Attaching the protective needle cap:
Attach the protective needle cap after step 5 (Setting up and using a new GoQuick syringe) to avoid needlestick injury.
- Remove the black plug from the protective needle cap. o If the needle guard slides out, push it back into the protective needle cap until it clicks into place.

and remove the needle.
- Align the black logo on the protective needle cap with the blue logo on the syringe. Carefully press the protective needle cap onto the syringe until it clicks.
- After step 6 (Setting up and using a new GoQuick syringe), press the black button to release the guard in the protective needle cap.
- Follow the instructions described in step 7 (Setting up and using a new GoQuick syringe).
To remove the needle with the protective needle cap attached:
- Place the outer needle shield on the end of the needle guard.
- Using the outer needle shield, push the needle guard until it clicks into place.
- Unscrew the needle using the shield and discard it into a suitable container for sharp waste.
- Leave the protective needle cap on the syringe.
- Put the black plug back onto the protective needle cap. Store the syringe in the refrigerator.
To remove the protective needle cap:
- First, remove the needle and then carefully slide the protective needle cap off the syringe.
- Do notdiscard the protective needle cap. It can be used with the next syringe.
GENOTROPIN 12 GOQUICK INSTRUCTIONS FOR USE
Read the entire instructions before using the GoQuick syringe. If you have any questions about the recommended dose or treatment with Genotropin 12, please contact your doctor or nurse.
Information about the GoQuick syringe
GoQuick is a pre-filled, multi-dose, single-use syringe containing 12 mg of somatropin. It can be used to administer Genotropin 12 in doses ranging from 0.3 mg to 4.5 mg. Each click of the black ring changes the dose by 0.15 mg. The Genotropin 12 in the syringe is mixed only once, at the first use of the syringe. The cartridges in the syringe are not interchangeable. After the syringe is empty, a new one should be used.
The syringe has a dose memory. The dose is set once in the syringe. Then, with each injection, it is set to administer the same dose. This prevents exceeding the set dose.
Important information
- Do notmix the powder and liquid in the syringe if there is no needle on it.
- Do notstore the syringe with the needle attached. Genotropin 12 may leak out, and air bubbles may form in the cartridge. Before preparing for storage, always remove the needle and attach the syringe cap or the protective needle cap.
- Be careful not to drop the syringe. If it is dropped and any part of it appears to be broken or damaged, do notuse it. Contact your doctor or nurse to obtain a new syringe. If the syringe is dropped but not damaged or broken, perform the dose reloading procedure according to the instructions in step 6 (Setting up and using a new GoQuick syringe).
- Clean the syringe with a damp cloth. Do notimmerse it in water.
- Use a new needle for each injection. Do notshare needles for syringes.
- The remaining volume scale on the side of the cartridge holder indicates the volume of Genotropin 12 remaining in the syringe.
Storage and disposal
- Store the syringe in the refrigerator (at a temperature between 2°C and 8°C), in the outer packaging, to protect it from light. Do notfreeze or expose to frost.
- Do notuse the syringe after the expiration date.
- After 28 days from mixing, discard the syringe, even if some medicine remains.
- Information on storing the GoQuick syringe can be found in this leaflet, on its second page.
- Dispose of the used syringe according to local regulations. In case of doubt, consult a doctor or nurse.
Components of the GoQuick syringe
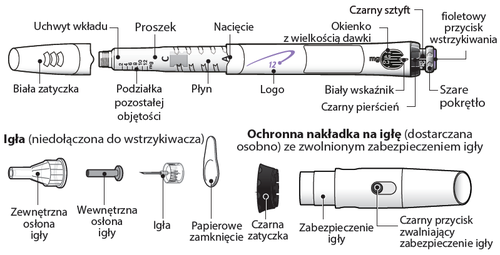
Needles are notincluded with the GoQuick syringe. Needles up to 8 mm in length should be purchased at a pharmacy.
- Needles for use with the GoQuick syringe: 31 G or 32 G (Becton Dickinson and Company) 31 G or 32 G (Novo Nordisk) 32.5 G or 34 G (Terumo)
Setting up and using a new GoQuick syringe
Step 1 Preparation

- Wash and dryyour hands.
- Gatherthe following materials and place them on a clean, flat surface: a new GoQuick syringe, a new needle (not included in the kit), and a suitable container for sharp waste (not included in the kit). Checkthe expiration date on the syringe label. Do notuse the syringe if the expiration date has passed.
Step 2 Choosing the injection site
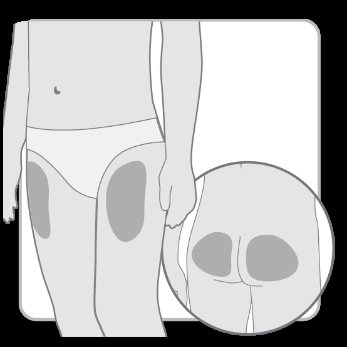
- Choose and cleanthe injection site according to the doctor's or nurse's recommendations. Choose a different site for each injection. Each subsequent injection should be performed at a site at least 2 cm away from the previous one.
- Avoid bony areas, bruised, red, painful, or hardened areas, as well as areas of skin covered with scars or affected by disease.
Step 3 Attaching a new needle

- Removethe white plug from the syringe.
- Take a new needle and remove the paper closure from it.
- Gently press and then screwthe needle onto the syringe. Do notovertighten. Caution:Be careful not to attach the needle at an angle. This may cause leakage.
- Leave both shields on the needle.
Step 4 Mixing Genotropin 12

- Holdthe syringe with the needle pointing upwards and the letter "A" towards you.
- Tightly screwthe cartridge holder into the syringe until you feel a click, when the letter "B" is in the notch.
- Gently tiltthe syringe from side to side to facilitate complete dissolution of the powder. Do notshake. Shaking may damage the growth hormone.
- Checkthat the liquid in the cartridge is clear and that all the powder has dissolved. o If the liquid is cloudy or powder is visible, gently tilt the syringe from side to side a few more times. o If the liquid is still cloudy or powder is visible, do notuse this syringe and try again with a new one.
Step 5 Removing air from the syringe

- Removethe outer needle shield. Keep it to secure the used needle after injection.
- Leave the inner needle shield in place. Caution:After removing the outer shield, the inner shield should be visible. If it is not visible, try to attach the needle again.
- Holdthe syringe with the needle pointing upwards.
- Gently tapthe cartridge to cause the trapped air to move upwards.
- Tightly screwthe cartridge holder into the syringe until you feel a click, when the letter "C" is in the notch. A small amount of liquid may appear around the inner needle shield. This is normal.
Step 6 Loading the dose
Loading the dose allows for the removal of any remaining air by expressing a small amount of liquid from the syringe. The loaded dose is 0.3 mg and differs from the dose prescribed by the doctor or nurse. This step should only be performed when using the syringe for the first time.
- Removeand discard the inner needle shield. Caution:Do not touch the needle to avoid pricking.
- Make surethat the dose of 0.3 mg is set in the dose window.
- Turnthe gray dial in the direction indicated by the arrows until it stops clicking.
- Holdthe syringe with the needle pointing straightupwards.
- Pressthe purple injection button until it stops clicking.
- Checkif there is liquid at the tip of the needle. If it appears, the syringe is ready for use. o If the liquid does not appear, repeat the steps described in the dose loading step, once or twice. o If the liquid still does not appear, do not use this syringe. Contact your doctor or nurse for advice.
Step 7 Setting and preparing the dose

When using the syringe for the first time, the dose prescribed by the doctor or nurse will be set.
There is no need to reset the dose until a new syringe is started or until the doctor or nurse advises to do so.
- Turn the black ring counterclockwiseuntil the recommended dose is aligned with the white pointer in the dose window. Be careful not to turn the gray dial.o If the selected dose is misaligned with the white pointer, turn the black ring in the other direction to set the correct dose value. Caution:If the black ring cannot be turned, press the purple injection button until it stops clicking. Then try to set the dose again. Remember that liquid will be expressed from the needle. Turn the gray dialin the direction indicated by the arrows until it stops clicking.
Step 8 Checking the dose
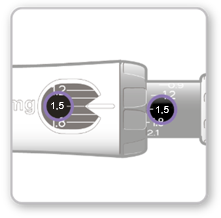
The selected dose value visible on the black rod should be alignedwith the white pointer.
- Make surethat the dose value visible on the black rod matches the value set in the dose window. o If these values are the same, the syringe is ready for injection. o If the dose values do not match, make sure the gray dial was turned in the correct direction indicated by the arrows until it stopped clicking.
Step 9 Injecting Genotropin 12
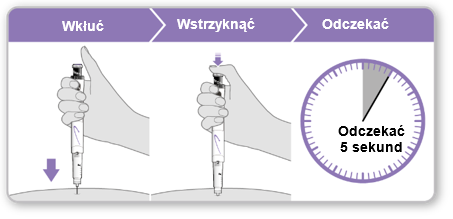
- Holdthe syringe over the injection site.
- Insertthe needle straight into the skin.
- Pressthe purple injection button until it stops clicking.
- Waitfor 5 full seconds to ensure the full dose is injected. During this time, maintain gentle pressure on the purple injection button.
- After 5 seconds, remove the needle from the skin without tilting it to the sides. Caution:If a drop of liquid is visible at the injection site or at the tip of the needle, during the next injection, hold the pressed purple injection button for a longer time before removing the needle from the skin.
Step 10 Removing the needle
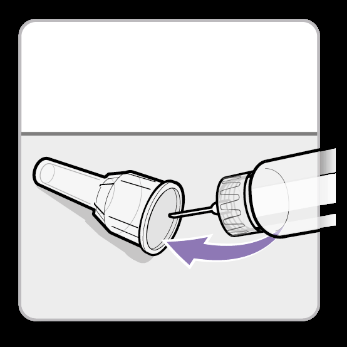
- Carefully slidethe outer shield onto the needle. Caution:Do not touch the needle to avoid pricking.
- Unscrewthe needle using the outer shield.
- Discardthe needle into a suitable container for sharp waste.
- Slidethe white plug onto the syringe.
- Store the syringe in the refrigerator until the next injection.
Regular (daily) use of the GoQuick syringe
Step 1 Preparation

- Wash and dryyour hands.
- Gatherthe following materials and place them on a clean, flat surface: the GoQuick syringe with the mixed medicine, a new needle (not included in the kit), and a suitable container for sharp waste (not included in the kit).
- Checkthe expiration date on the syringe label. Do notuse the syringe if the expiration date has passed. Do notuse the syringe after 28 days from the first use.
Step 2 Choosing the injection site
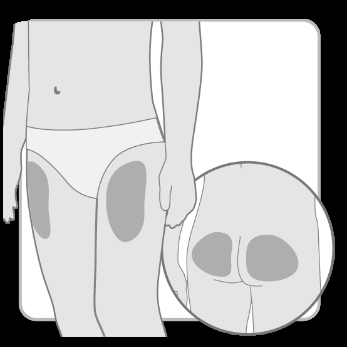
Choose and cleanthe injection site according to the doctor's or nurse's recommendations. Choose a different site for each injection. Each subsequent injection should be performed at a site at least 2 cm away from the previous one.
Avoid bony areas, bruised, red, painful, or hardened areas, as well as areas of skin covered with scars or affected by disease.
Step 3 Attaching a new needle
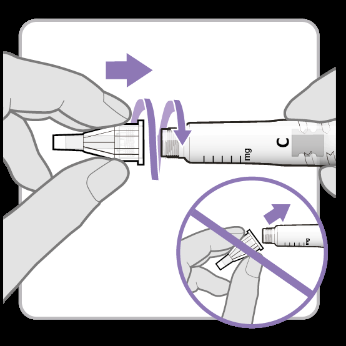
- Removethe white plug from the syringe.
- Take a new needle and remove the paper closure from it.
- Gently press and then screwthe needle onto the syringe. Do notovertighten. Caution:Be careful not to attach the needle at an angle. This may cause leakage.
- Removeboth shields from the needle. Keep the outer needle shield to secure the needle after injection.
Step 4 Loading the dose

- Turn the gray dialin the direction indicated by the arrows until it stops clicking.
- The selected dose value visible on the black rod should be alignedwith the white pointer.
- Make surethat the dose value visible on the black rod matches the value set in the dose window. o If these values are the same, the syringe is ready for injection. Caution:If the prepared dose is smaller, it means that the syringe does not contain a full dose of Genotropin 12. If the syringe does not contain a full dose, follow the doctor's or nurse's instructions or contact them for advice.
If the syringe does not contain a full dose, follow the doctor's or nurse's instructions or contact them for advice.
Step 5 Injecting Genotropin 12
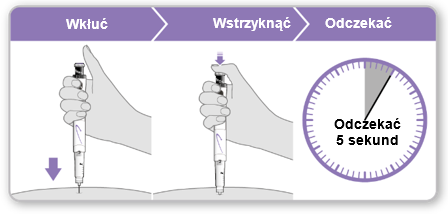
- Holdthe syringe over the injection site.
- Insertthe needle straight into the skin.
- Pressthe purple injection button until it stops clicking.
- Waitfor 5 full seconds to ensure the full dose is injected. During this time, maintain gentle pressure on the purple injection button.
- After 5 seconds, remove the needle from the skin without tilting it to the sides. Caution:If a drop of liquid is visible at the injection site or at the tip of the needle, during the next injection, hold the pressed purple injection button for a longer time before removing the needle from the skin.
Step 6 Removing the needle
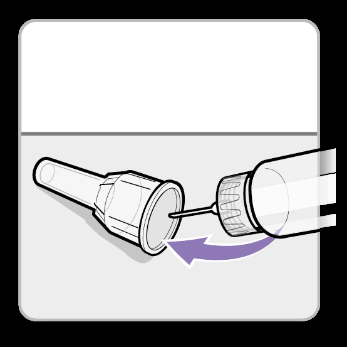
- Carefully slidethe outer shield onto the needle. Caution:Do not touch the needle to avoid pricking.
- Unscrewthe needle using the outer shield.
- Discardthe needle into a suitable container for sharp waste.
- Slidethe white plug onto the syringe.
- Store the syringe in the refrigerator until the next injection.
Using the Protective Needle Cap (Optional)
The protective needle cap is an optional component supplied separately, which allows you to conceal the needle while administering the injection.
Attaching the Protective Needle Cap:
The protective needle cap should be attached after completing step 5 (Setting up and using a new GoQuick injector), to avoid needle stick injury.
- Remove the black cap from the protective needle cap. If the needle shield slides out, push it back into the protective cap until it clicks into place.

until it clicks into place.
- Align the black logo on the protective needle cap with the purple logo on the injector. Carefully push the protective cap onto the injector until it clicks.
- After completing step 6 (Setting up and using a new GoQuick injector), press the black button to release the lock on the protective needle cap.
- Follow the instructions described in step 7 (Setting up and using a new GoQuick injector).
To Remove the Needle with the Protective Needle Cap Attached:
- Place the outer needle shield over the end of the needle shield.
- Using the outer needle shield, push the needle shield until it locks into place.
- Using the shield, unscrew the needle and dispose of it in an appropriate sharps container.
- Leave the protective needle cap on the injector.
- Put the black cap back on the protective needle cap. Store the injector in the refrigerator.
To Remove the Protective Needle Cap:
- First, remove the needle, then carefully pull the protective needle cap off the injector.
- Do notdiscard the protective needle cap. It can be used with the next injector.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterPfizer Health AB Pfizer Manufacturing Belgium NV
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Genotropin 5,3Dosage form: Powder, 12 mg (36 IU)Active substance: somatropinManufacturer: Pfizer Manufacturing Belgium NVPrescription requiredDosage form: Solution, 10 mg/1.5 mlActive substance: somatropinPrescription requiredDosage form: Solution, 5 mg/1.5 mlActive substance: somatropinPrescription required
Alternatives to Genotropin 5,3 in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Genotropin 5,3 in Spain
Alternative to Genotropin 5,3 in Ukraine
Online doctors for Genotropin 5,3
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Genotropin 5,3 – subject to medical assessment and local rules.










