
GENOTONORM MINIQUICK 0.2 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use GENOTONORM MINIQUICK 0.2 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
GENOTONORM MINIQUICK 0.2 mg, 0.4 mg, 0.6 mg, 0.8 mg, 1.0 mg, 1.2 mg, 1.4 mg, 1.6 mg, 1.8 mg, 2.0 mgpowder and solvent for solution for injection
somatropin
Read all of this leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
Contents of the package leaflet:
- What is Genotonorm Miniquick and what is it used for
- What you need to know before you start using Genotonorm Miniquick
- How to use Genotonorm Miniquick
- Possible side effects
- Storage of Genotonorm Miniquick
- Contents of the pack and other information
1. What is Genotonorm Miniquick and what is it used for
Genotonorm Miniquick is a recombinant human growth hormone (also known as somatropin). It has the same structure as human growth hormone, which is necessary for bone and muscle growth. It also helps fat and muscle tissue develop in the right amounts. The fact that it is recombinant means that it does not come from human or animal tissue.
In children, Genotonorm Miniquick is used to treat growth disorders:
- If they are not growing properly or do not have enough growth hormone of their own.
- In the case of Turner syndrome. Turner syndrome is a chromosomal disorder that affects girls and can affect growth – your doctor will tell you if you have it.
- In the case of chronic renal failure. If the kidney loses its ability to function properly, growth can be affected.
- In the case of Prader-Willi syndrome (a chromosomal disorder). Growth hormone will help you grow if you are still in the growth phase and will also improve body composition. Excess fat will decrease and muscle loss will improve.
- If you were born small or with low birth weight. Growth hormone can help you grow if you have not been able to reach or maintain normal growth by the age of 4 or later.
In adults, Genotonorm Miniquick is used to treat people with a marked deficiency of growth hormone. This can start in adulthood or may have started in childhood and continued into adulthood.
If you have been treated with Genotonorm Miniquick for a growth hormone deficiency during childhood, your growth hormone levels should be re-evaluated after completing the growth phase. If a severe growth hormone deficiency is confirmed, your doctor will propose continuing treatment with Genotonorm Miniquick.
This medicine can only be prescribed by a doctor with experience in growth hormone treatment and who has confirmed your diagnosis.
2. What you need to know before you start using Genotonorm Miniquick
Do not use Genotonorm Miniquick and contact your doctor if
- You are allergic (hypersensitive) to somatropin or any of the other ingredients of Genotonorm Miniquick.
- You have an active tumor (cancer). Tumors must be inactive and you must have finished anti-tumor treatment before starting treatment with Genotonorm.
- You are seriously ill (for example, complications after open-heart surgery, abdominal surgery, acute respiratory failure, accidental trauma, or a similar situation). If you are about to undergo or have undergone major surgery or are going to be hospitalized for any reason, tell your doctor and remind other doctors who examine you that you are using growth hormone.
- You have already finished your growth phase (epiphyses closed) and you were prescribed Genotonorm Miniquick to stimulate growth.
Be careful with Genotonorm Miniquick and contact your doctor
- If you are at risk of developing diabetes, your doctor will monitor your blood sugar levels during treatment with Genotonorm Miniquick.
- If you have diabetes, you should carefully monitor your blood sugar levels during treatment with Genotonorm Miniquick and review the results with your doctor to determine if you need to change the dose of your diabetes medications.
- After starting treatment with Genotonorm, some patients may need to start treatment with thyroid hormone.
- If you are receiving treatment with thyroid hormones, it may be necessary to adjust the dose of thyroid hormone.
- If you are receiving growth hormone treatment to stimulate growth and you start limping, or if you start limping because you have hip pain during growth hormone treatment, you should inform your doctor.
- If you experience an increase in intracranial pressure (with symptoms such as severe headaches, vision problems, or vomiting), you should inform your doctor.
- If you are receiving Genotonorm Miniquick for a growth hormone deficiency after a previous tumor (cancer), you should undergo regular review for possible tumor recurrences or any other cancer.
- If you experience abdominal pain that worsens, you should inform your doctor.
- Experience in patients over 80 years of age is limited. Elderly people may be more sensitive to the action of Genotonorm Miniquick and therefore may be more likely to develop side effects.
Children with chronic renal failure:
- Your doctor will examine your kidney function and growth rate before starting treatment with Genotonorm Miniquick. Medical treatment for your kidney disease should continue. Treatment with Genotonorm Miniquick should be suspended in case of kidney transplant.
Children with Prader-Willi syndrome:
- Your doctor will give you dietary restrictions to control your weight.
- Your doctor will perform an examination before starting treatment with Genotonorm Miniquick to determine if you have upper airway obstruction, sleep apnea (when breathing stops during sleep), or respiratory infections.
- If you develop signs of upper airway obstruction (including the onset or increase of snoring) during treatment, your doctor will need to examine you and may interrupt treatment with Genotonorm Miniquick.
- During treatment, your doctor will monitor any signs of scoliosis, a type of spinal deformity.
- If you develop a lung infection during treatment, tell your doctor so that they can treat the infection.
Children born small or with low birth weight:
- If you were born small or with low birth weight and are between 9 and 12 years old, consult your doctor specifically about puberty and treatment with this product.
- Your doctor will perform blood sugar and insulin tests before starting treatment and once a year while it continues.
- Treatment should continue until the growth phase is complete.
Use in athletes
This medicine contains somatropin, which can produce a positive result in doping tests.
Use of other medicines
Tell your doctor or pharmacist if you are using or have recently used any other medicines, including those obtained without a prescription.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Genotonorm.
If you are receiving replacement treatment with glucocorticoids, you should consult your doctor regularly as it may be necessary to adjust your glucocorticoid dose.
Tell your doctor if you are using:
- medicines for the treatment of diabetes
- thyroid hormones
- synthetic adrenal hormones (corticosteroids)
- estrogens administered orally or other sex hormones
- cyclosporin (a medicine that weakens the immune system after a transplant)
- medicines for the control of epilepsy (anticonvulsants)
Your doctor may need to adjust the dose of these medicines or the dose of Genotonorm Miniquick.
Pregnancy and breastfeeding
Do not use Genotonorm if you are pregnant, think you may be pregnant, or are planning to become pregnant.
Consult your doctor before using this medicine while breastfeeding.
Consult your doctor or pharmacist before using any medicine.
Genotonorm Miniquick contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; it is essentially "sodium-free".
3. How to use Genotonorm Miniquick
Recommended dose
The dose depends on your body surface area, the condition for which you are being treated, and how your growth hormone is working. Each person is different. Your doctor will tell you your individualized dose of Genotonorm Miniquick in milligrams (mg) based on your body weight in kilograms (kg) or your body surface area calculated from your height and weight in square meters (m2), as well as your treatment schedule. Do not change the dose or treatment schedule without consulting your doctor.
Children with growth hormone deficiency:
0.025-0.035 mg/kg body weight per day or 0.7-1.0 mg/m2 body surface area per day. Higher doses may be used. When the growth hormone deficiency continues into adolescence, treatment with Genotonorm should continue until physical development is complete.
Children with Turner syndrome:
0.045-0.050 mg/kg body weight per day or 1.4 mg/m2 body surface area per day.
Children with chronic renal failure:
0.045-0.050 mg/kg body weight per day or 1.4 mg/m2 body surface area per day. If the growth rate is too low, higher doses may be necessary. A dose adjustment may be necessary after 6 months of treatment.
Children with Prader-Willi syndrome:
0.035 mg/kg body weight per day or 1.0 mg/m2 body surface area per day. The daily dose should not exceed 2.7 mg. This treatment should not be used in children whose growth phase has almost finished after puberty.
Children born small or with low birth weight and with growth disorders:
0.035 mg/kg body weight per day or 1.0 mg/m2 body surface area per day. It is important to continue treatment until final height is reached. Treatment should be suspended after the first year if there is no response or if final height is reached and growth has stopped.
Adults with growth hormone deficiency:
If you continue to use Genotornorm Miniquick after treatment during childhood, you should start with a dose of 0.2-0.5 mg per day. This dose should be gradually increased or decreased according to the analytical results, as well as the clinical response and side effects.
If the growth hormone deficiency starts during adulthood, you should start with 0.15-0.3 mg per day. This dose should be gradually increased based on the analytical results, as well as the clinical response and side effects. The daily maintenance dose rarely exceeds 1.0 mg per day. Women may require higher doses than men. The dosage should be monitored every 6 months. Patients over 60 years of age should start with doses of 0.1-0.2 mg per day and gradually increase as needed. The minimum effective dose should be used. The maintenance dose rarely exceeds 0.5 mg per day. Follow the instructions indicated by your doctor.
Injection of Genotonorm Miniquick
Genotonorm Miniquick is used subcutaneously. This means that it is injected through a small needle into the fatty tissue, just under the skin. Your doctor will teach you how to use Genotonorm Miniquick. Always use Genotonorm Miniquick as your doctor has indicated. Consult your doctor or pharmacist if you have any doubts.
Please read the "Instructions for use" at the end of this leaflet for information on how to use Genotonorm Miniquick. If you cannot remember how to do it, do not attempt to give yourself the injection. Ask your doctor to teach you again.
You can take the growth hormone out of the refrigerator 30 minutes before the injection. This allows it to warm up a bit and makes the injection more comfortable.
Remember to wash your hands and clean your skin before injecting yourself.
Give yourself the growth hormone injection at the same time every day. A good time is bedtime, as it is easy to remember. It is also normal to have a higher level of growth hormone at night.
Most patients use the thighs or buttocks for the injection. Give yourself the injection where your doctor has taught you. The fatty tissue under the skin can decrease in size at the injection site. To avoid this, change the injection site each time. This will give your skin and the area under the skin time to recover between injections before injecting again in the same spot.
If you use more Genotonorm Miniquick than you should
If you inject more than you should, consult your doctor or pharmacist immediately. Blood sugar levels can drop sharply and then rise to very high levels. You may feel agitated, sweaty, sleepy, or strange, and you may feel dizzy.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 5620420, indicating the medicine and the amount ingested.
If you forget to administer Genotonorm Miniquick
Do not administer a double dose to make up for forgotten doses.
It is best to inject growth hormone regularly. If you forget to administer a dose, give yourself the next injection at the corresponding time the next day. Note the forgotten injections and tell your doctor at the next review.
If you interrupt treatment with Genotonorm Miniquick
Consult your doctor before interrupting treatment with Genotonorm Miniquick.
If you have any other questions about the use of this product, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, Genotonorm Miniquick can cause adverse effects, although not all people suffer from them.
Frequent and very frequent adverse effects in adults may start in the first few months of treatment and disappear spontaneously or when the dose is reduced.
Very frequent adverse effects (may affect more than 1 in 10 patients) include:
In adults:
- Joint pain.
- Fluid retention (manifested as swollen fingers or ankles).
Frequent adverse effects (may affect up to 1 in 10 patients) include:
In children:
- Joint pain.
- Redness, itching, or temporary pain at the injection site.
In adults:
- Numbness/tingling.
- Pain or burning sensation in the hands or armpits (known as carpal tunnel syndrome).
- Stiffness in arms and legs, muscle pain.
Uncommon adverse effects (may affect up to 1 in 100 patients) include:
In children:
- Leukemia (reported in a small number of patients with growth hormone deficiency, some of whom received somatropin treatment. However, there is no evidence that the incidence of leukemia is increased in growth hormone recipients without predisposing factors).
- Increased intracranial pressure (causing symptoms such as severe headache, vision problems, or vomiting).
- Numbness/tingling.
- Rash.
- Itching.
- Hives on the skin with itching.
- Muscle pain.
- Enlargement of the breast (gynecomastia).
- Fluid retention (manifested as swollen fingers or ankle swelling, during a short period at the start of treatment).
In adults:
- Enlargement of the breast (gynecomastia).
Frequency not known: cannot be estimated from available data:
- Type II diabetes.
- Swelling of the face.
- Headache.
- Decreased cortisol hormone levels in the blood.
In children:
- Stiffness in arms and legs.
In adults:
- Increased intracranial pressure (causing symptoms such as severe headache, vision problems, or vomiting).
- Rash.
- Itching.
- Hives on the skin with itching.
- Redness, itching, or pain at the injection site.
Formation of antibodies against the injected growth hormone, although this does not seem to affect the action of the growth hormone.
The skin around the injection site may become rough and irregular, but this should not happen if the injection is given at a different site each time.
There have been rare cases of sudden death in patients with Prader-Willi syndrome. However, no relationship has been established between these cases and treatment with Genotonorm Miniquick.
If you experience discomfort or pain in the hip or knee while receiving treatment with Genotonorm, your doctor may consider the possibility that you suffer from slipped capital femoral epiphysis or Legg-Calvé-Perthes disease.
Other possible side effects related to growth hormone treatment are as follows.
You (or your child) may experience increased blood sugar levels or decreased thyroid hormone levels. Your doctor may perform tests to determine this and, if necessary, prescribe the appropriate treatment. Occasionally, pancreatitis has been reported in patients treated with growth hormone.
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the Spanish Medicines Monitoring System: https://www.notificaRAM.es.
By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Genotonorm Miniquick
Keep out of sight and reach of children.
Do not use this medicine after the expiration date stated on the packaging as MM/YYYY. The expiration date is the last day of the month indicated.
Before reconstitution
Store in a refrigerator (2°C-8°C). Do not freeze. Keep the syringe in the outer packaging to protect it from light.
Before opening, the product can be stored outside the refrigerator for a maximum of 6 months at a temperature not exceeding 25°C. The date on which the medicine is removed from the refrigerator and the new expiration date must be noted on the outer packaging. This new expiration date must never exceed the date initially indicated on the outer packaging. If you have not used the medicine before the new expiration date, you must discard it.
After reconstitution
Use immediately or store in a refrigerator (2°C-8°C) for a maximum of 24 hours. Do not freeze. Keep the syringe in the outer packaging to protect it from light.
Do not use this medicine if you notice particles or if the solution is not transparent.
Never throw away needles or empty syringes in the regular trash. When you have finished using the needle, you must dispose of it carefully in a special container for needles, so that no one can use it or prick themselves.
Medicines should not be thrown away in drains or trash. Deposit the packaging and medicines you no longer need at the SIGRE collection point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Package Contents and Additional Information
Composition of Genotonorm Miniquick
- The active ingredient is somatropin*.
- A vial contains 0.2 mg, 0.4 mg, 0.6 mg, 0.8 mg, 1.0 mg, 1.2 mg, 1.4 mg, 1.6 mg, 1.8 mg, or 2.0 mg of somatropin* in 0.25 ml after reconstitution, which corresponds to a concentration of 0.8 mg, 1.6 mg, 2.4 mg, 3.2 mg, 4.0 mg, 4.8 mg, 5.6 mg, 6.4 mg, 7.2 mg, and 8.0 mg/ml.
- The other components of the powder are: glycine (E640), mannitol (E421), sodium phosphate anhydrous (E339), and disodium dihydrogen phosphate anhydrous (E339) (see section 2 "Genotonorm Miniquick contains sodium").
- The ingredients of the solvent are: water for injectable preparations and mannitol (E421).
*Obtained in Escherichia colicells by recombinant DNA technology.
Appearance of Genotonorm Miniquickand package contents
Powder and solvent for solution for injection, in a dual-chamber vial containing the powder in one section and the solvent in the other (0.2mg/0.25 ml, 0.4mg/0.25 ml, 0.6mg/0.25 ml, 0.8mg/0.25 ml, 1.0 mg/0.25 ml, 1.2mg/0.25 ml, 1.4 mg/0.25 ml, 1.6 mg/0.25 ml, 1.8 mg/0.25 ml or 2.0 mg/0.25ml). The vial is contained in a syringe. Package sizes: 4, 7, or 28 syringes.
Only some package sizes may be marketed.
The powder is white and the solvent is transparent.
Marketing Authorization Holder
Pfizer, S.L.
Avda. de Europa 20-B
Parque Empresarial La Moraleja
28108 Alcobendas (Madrid), Spain.
Manufacturer
Pfizer Manufacturing Belgium NV
Rijksweg 12
2870
Puurs-Sint-Amands
Belgium
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Genotropin Miniquick: Austria, Denmark, Germany, Greece, Ireland, Italy, Portugal, Sweden, United Kingdom (Northern Ireland).
Genotonorm Miniquick: Belgium, France, Luxembourg, Spain.
Date of the last revision of this leaflet:May 2024
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
INSTRUCTIONS FOR USE OF GENOTONORM MINIQUICK
Genotonorm Miniquick is a syringe used to mix and administer a single dose of Genotonorm (growth hormone).
Genotonorm Miniquick is supplied preloaded with a dual-chamber vial and a needle. If you need additional needles, ask for the same Becton Dickinson Micro-fine needles that are supplied with Miniquick. The injection volume is always 0.25 ml.
Genotonorm Miniquick is disposable; after administering the dose, discard it as described in step 6.
The diagram below identifies its different components.
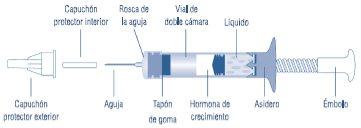
The Genotonorm Miniquick vial contains the growth hormone powder in one chamber and the solvent in the other. When the plunger is turned clockwise, the growth hormone powder and solvent mix and the powder dissolves.
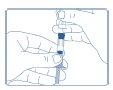
- Remove the paper covering the injection needle. Place the needle firmly until it hits the rubber stopper. Turn the Genotonorm Miniquick needle clockwise until it cannot turn anymore.
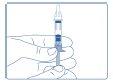
- Hold Genotonorm Miniquick with the needle facing up. Turn the plunger clockwise until it reaches the stop.
DO NOT SHAKE the solution. Mix gently. If you shake the solution, you may create foam with the growth hormone and damage the active ingredient. Check that the solution is transparent and use only transparent and particle-free solutions.
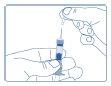
- Remove the outer and inner caps of the needle.
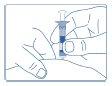
- Firmly pinch the area where the injection will be given and insert the needle.
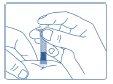
- Inject by pressing the plunger as far as possible to inject all the contents of Genotonorm Miniquick. Wait a few seconds before removing the needle, to allow enough time for all the growth hormone to be injected.
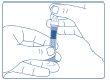
- After the injection, do not attempt to cover the needle with the cap. Discard the syringe with the needle, and the outer and inner caps, following the usual procedures or the instructions given by your doctor or pharmacist.
QUESTIONS AND ANSWERS
Question Is it a problem if I see air bubbles in the syringe? What should I do if there is resistance when turning the plunger (step 2) or when giving the injection (step 5)? What should I do if the needle is damaged or bent? | Answer No. It is not necessary to remove the air from Genotonorm Miniquick. The small amount of air in the syringe does not affect the injection. Resistance may be due to the needle being inserted crookedly over the rubber stopper. Carefully place the protective outer cap (the white opaque one) over the needle and turn it counterclockwise to remove the needle. Hold the Miniquick syringe with the end where the needle is placed facing up and place the needle straight onto the end of the syringe. Screw the needle onto the syringe. Discard the needle and use a new one with Miniquick. |
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to GENOTONORM MINIQUICK 0.2 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTIONDosage form: INJECTABLE, 12 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription requiredDosage form: INJECTABLE, 5.3 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription requiredDosage form: INJECTABLE, 0.4 mg somatropinActive substance: somatropinManufacturer: Pfizer S.L.Prescription required
Online doctors for GENOTONORM MINIQUICK 0.2 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Discuss questions about GENOTONORM MINIQUICK 0.2 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











