
GENOTONORM KABIPEN 12 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION

How to use GENOTONORM KABIPEN 12 mg POWDER AND SOLVENT FOR INJECTABLE SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
GENOTONORM KABIPEN 5.3 mg and 12 mg powder and solvent for solution for injection
somatropin
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet.
Contents of the pack:
- What is Genotonorm Kabipen and what is it used for
- What you need to know before you use Genotonorm Kabipen
- How to use Genotonorm Kabipen
- Possible side effects
- Storage of Genotonorm Kabipen
- Contents of the pack and other information
1. What is Genotonorm Kabipen and what is it used for
Genotonorm Kabipen is a recombinant human growth hormone (also known as somatropin). It has the same structure as human growth hormone, which is necessary for bone and muscle growth. It also helps fat and muscle tissue develop in the right amounts. The fact that it is recombinant means that it does not come from human or animal tissue.
In children, Genotonorm Kabipen is used to treat growth disorders:
- If they are not growing properly or if they do not have enough growth hormone of their own.
- In the case of Turner syndrome. Turner syndrome is a chromosomal disorder that affects girls and can affect growth – your doctor will tell you if you have it.
- In the case of chronic kidney failure. If the kidney loses its ability to function properly, growth can be affected.
- In the case of Prader-Willi syndrome (a chromosomal disorder). Growth hormone will help you grow if you are still in the growth phase and will also improve body composition. Excess fat will decrease and muscle loss will improve.
- If you were born small or with low weight. Growth hormone can help you grow if you have not been able to reach or maintain normal growth by the age of 4 or later.
In adults, Genotonorm Kabipen is used to treat people with a marked deficiency of growth hormone. This can start in adulthood or may have started in childhood and continue into adulthood.
If you have been treated with Genotonorm Kabipen for a growth hormone deficiency during childhood, your growth hormone levels should be re-evaluated after completing the growth phase. If a severe growth hormone deficiency is confirmed, your doctor will suggest continuing treatment with Genotonorm Kabipen.
This medicine can only be prescribed by a doctor with experience in growth hormone treatment and who has confirmed your diagnosis.
2. What you need to know before you use Genotonorm Kabipen
Do not use Genotonorm Kabipen and contact your doctor if
- You are allergic (hypersensitive) to somatropin or any of the other components of Genotonorm Kabipen.
- You have an active tumor (cancer). Tumors must be inactive and anti-tumor treatment must have been completed before starting treatment with Genotonorm.
- You are seriously ill (for example, complications after open-heart surgery, abdominal surgery, acute respiratory failure, accidental trauma, or a similar situation). If you are about to undergo or have undergone major surgery or are going to be hospitalized for any reason, tell your doctor and remind other doctors who examine you that you are using growth hormone.
- You have already finished your growth phase (epiphyses closed) and you were prescribed Genotonorm Kabipen to stimulate growth.
Be careful with Genotonorm Kabipen and contact your doctor
- If you are at risk of developing diabetes, your doctor will monitor your blood sugar levels during treatment with Genotonorm Kabipen.
- If you have diabetes, you should carefully monitor your blood sugar levels during treatment with Genotonorm Kabipen and review the results with your doctor to determine if you need to change the dose of your diabetes medications.
- After starting treatment with Genotonorm Kabipen, some patients may need to start treatment with thyroid hormone.
- If you are receiving treatment with thyroid hormones, it may be necessary to adjust the dose of thyroid hormone.
- If you are receiving growth hormone treatment to stimulate growth and you limp, or if you start limping because you have hip pain during growth hormone treatment, you should inform your doctor.
- If you experience an increase in intracranial pressure (with symptoms such as severe headaches, vision problems, or vomiting), you should inform your doctor.
- If your doctor confirms that you have developed muscle inflammation near the injection site due to the preservative metacresol, you should use another presentation of Genotonorm that does not contain metacresol.
- If you are receiving Genotonorm Kabipen for a growth hormone deficiency after a previous tumor (cancer), you should undergo periodic review for possible tumor recurrences or any other cancer.
- If you experience abdominal pain that worsens, you should inform your doctor.
- Experience in patients over 80 years of age is limited. Elderly people may be more sensitive to the action of Genotonorm Kabipen and may be more prone to developing side effects.
Children with chronic kidney failure:
- Your doctor will examine your kidney function and growth rate before starting treatment with Genotonorm Kabipen. Medical treatment for your kidney disease should continue. Treatment with Genotonorm Kabipen should be suspended in case of kidney transplant.
Children with Prader-Willi syndrome:
- Your doctor will give you dietary restrictions to control your weight.
- Your doctor will perform an examination before starting treatment with Genotonorm Kabipen to determine if you have upper airway obstruction, sleep apnea (when breathing stops during sleep), or respiratory infections.
- If during treatment you present signs of upper airway obstruction (including the onset or increase of snoring), your doctor will need to examine you and may interrupt treatment with Genotonorm Kabipen.
- During treatment, your doctor will monitor any signs of scoliosis, a type of spinal deformity.
- If during treatment you develop a lung infection, tell your doctor so that they can treat the infection.
Children born small or with low weight:
- If you were born small or with low weight and are between 9 and 12 years old, consult your doctor specifically about puberty and treatment with this product.
- Your doctor will perform blood sugar and insulin tests before starting treatment and once a year while it lasts.
- Treatment should continue until the growth phase is complete.
Use in athletes
This medicine contains somatropin, which can produce a positive result in doping tests.
Use of other medicines
Tell your doctor or pharmacist if you are using or have recently used any other medicines, including those obtained without a prescription.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Genotonorm.
If you are receiving replacement treatment with glucocorticoids, you should consult your doctor regularly as it may be necessary to adjust your glucocorticoid dose.
Tell your doctor if you are using:
- medicines for the treatment of diabetes
- thyroid hormones
- synthetic adrenal hormones (corticosteroids)
- estrogens administered orally or other sex hormones
- cyclosporin (a medicine that weakens the immune system after a transplant)
- medicines for the control of epilepsy (anticonvulsants)
Your doctor may need to adjust the dose of these medicines or the dose of Genotonorm Kabipen.
Pregnancy and breastfeeding
Do not use Genotonorm Kabipen if you are pregnant, think you may be pregnant, or are planning to become pregnant.
Consult your doctor before using this medicine while breastfeeding.
Consult your doctor or pharmacist before using any medicine.
Genotonorm Kabipen contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per dose; it is essentially "sodium-free".
3. How to use Genotonorm Kabipen
Recommended dose
The dose depends on your body surface area, the condition for which you are being treated, and how your growth hormone is functioning. Each person is different. Your doctor will indicate your individualized dose of Genotonorm Kabipen in milligrams (mg) based on your body weight in kilograms (kg) or your body surface area calculated from your height and weight in square meters (m2), as well as the treatment schedule. Do not change the dose or treatment schedule without consulting your doctor.
Children with growth hormone deficiency:
0.025-0.035 mg/kg body weight per day or 0.7-1.0 mg/m2 body surface area per day. Higher doses may be used. When the growth hormone deficiency continues into adolescence, treatment with Genotonorm Kabipen should continue until physical development is complete.
Children with Turner syndrome:
0.045-0.050 mg/kg body weight per day or 1.4 mg/m2 body surface area per day.
Children with chronic kidney failure:
0.045-0.050 mg/kg body weight per day or 1.4 mg/m2 body surface area per day. If the growth rate is too low, higher doses may be necessary. A dose adjustment may be necessary after 6 months of treatment.
Children with Prader-Willi syndrome:
0.035 mg/kg body weight per day or 1.0 mg/m2 body surface area per day. The daily dose should not exceed 2.7 mg. This treatment should not be used in children whose growth phase has almost finished after puberty.
Children born small or with low weight and with growth disorders:
0.035 mg/kg body weight per day or 1.0 mg/m2 body surface area per day. It is important to continue treatment until the final height is reached. Treatment should be suspended after the first year if there is no response or if the final height is reached and growth has stopped.
Adults with growth hormone deficiency:
If you continue using Genotornorm after treatment during childhood, you should start with a dose of 0.2-0.5 mg per day. This dose should be gradually increased or decreased according to the analytical results, as well as the clinical response and side effects.
If the growth hormone deficiency starts during adulthood, you should start with 0.15-0.3 mg per day. This dose should be gradually increased based on the analytical results and the clinical response and side effects. The daily maintenance dose rarely exceeds 1.0 mg per day. Women may require higher doses than men. The dose should be monitored every 6 months. Patients over 60 years of age should start with a dose of 0.1-0.2 mg per day and gradually increase it according to individual needs. The minimum effective dose should be used. The maintenance dose rarely exceeds 0.5 mg per day. Follow the instructions indicated by your doctor.
Injection of Genotonorm Kabipen
This medicine is used subcutaneously. This means that it is injected through a small needle into the fatty tissue, just under the skin. Your doctor will teach you how to use it. Always use the medicine as your doctor has indicated. Consult your doctor or pharmacist if you have any doubts.
The instructions for use of the pre-filled device GoQuick are also provided in the box of the pre-filled device.
The instructions for using Genotonorm Kabipen in a dual-chamber vial with Genotonorm Pen are provided in the injection device case.
Consult these instructions before using your medicine.
When using a pre-filled device or a Genotonorm Pen injection device, you should place the needle on the device before mixing. Use a new needle with each injection. Needles should not be reused.
- Preparation of the injection:
You can take the medicine out of the refrigerator 30 minutes before the injection. This allows it to warm up a bit and makes the injection more comfortable.
The pre-filled device GoQuick contains a dual-chamber vial that has the growth hormone and the liquid solvent. The growth hormone and the liquid solvent are mixed by turning the vial (see the steps in detail in the Instructions for Use). No additional device is needed.
Genotonorm is also available in a dual-chamber vial that contains the growth hormone and the liquid solvent for use with the Genotonorm Pen device. The growth hormone and the liquid solvent in the dual-chamber vial can be mixed by screwing the Genotonorm Pen device together.
In both cases, for the pre-filled device GoQuick and the dual-chamber vial, dissolve the powder by gently tilting it until the powder is completely dissolved.
When mixing Genotonorm Kabipen, DO NOT SHAKE the solution. Mix it gently. If you shake the solution, you can cause the growth hormone to foam and damage the medicine. Check the solution and do not inject it if you notice turbidity or particles in it.
- Injection of Genotonorm Kabipen:
Remember to wash your hands first and clean the skin at the injection site.
Give yourself the growth hormone injection at the same time every day. A good time is bedtime, as it is easy to remember. It is also normal to have a higher level of growth hormone at night.
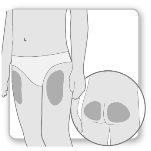
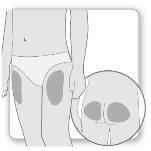
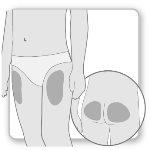
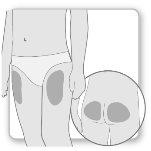
Most patients use the thighs or buttocks for the injection. Give yourself the injection where your doctor has taught you. The fatty tissue of the skin may decrease in size at the injection site. To avoid this, change the injection site each time. This will give your skin and the area under the skin time to recover between injections before injecting again in the same spot.
Remember to store the medicine in the refrigerator immediately after the injection.
If you use more Genotonorm Kabipen than you should
If you inject more than you should, consult your doctor or pharmacist immediately. Blood sugar levels may drop sharply and then rise to excessively high levels. You may feel agitated, sweaty, sleepy, or strange, and you may become dizzy.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 5620420, indicating the medicine and the amount ingested.
If you forget to administer Genotonorm Kabipen
Do not administer a double dose to make up for forgotten doses.
It is best to inject growth hormone regularly. If you forget to administer a dose, give yourself the next injection at the corresponding time the next day. Note the forgotten injections and tell your doctor at the next review.
If you interrupt treatment with Genotonorm Kabipen
Consult your doctor before interrupting treatment with this medicine.
If you have any other questions about the use of this product, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them.
Common and very common adverse effects in adults may start in the first few months of treatment and disappear spontaneously or when the dose is reduced.
The very common adverse effects (may affect more than 1 in 10 patients) include:
In adults:
- Joint pain.
- Fluid retention (which manifests as swollen fingers or ankle swelling).
The common adverse effects (may affect up to 1 in 10 patients) include:
In children:
- Joint pain.
- Redness, itching, or temporary pain at the injection site.
In adults:
- Numbness/tingling.
- Pain or burning sensation in the hands or armpits (known as carpal tunnel syndrome).
- Stiffness in arms and legs, muscle pain.
The uncommon adverse effects (may affect up to 1 in 100 people) include:
In children:
- Leukemia (has been reported in a small number of patients with growth hormone deficiency, some of whom have received somatropin treatment. However, there is no evidence that the incidence of leukemia is increased in growth hormone recipients without predisposing factors).
- Increased intracranial pressure (which causes symptoms such as severe headache, vision problems, or vomiting).
- Numbness/tingling.
- Rash.
- Itching.
- Hives on the skin with itching.
- Muscle pain.
- Enlargement of the breast (gynecomastia).
- Fluid retention (which manifests as swollen fingers or ankle swelling, for a short period at the beginning of treatment).
In adults:
- Enlargement of the breast (gynecomastia).
Frequency not known: cannot be estimated from the available data:
- Type 2 diabetes.
- Swelling of the face.
- Headache.
- Decreased cortisol hormone levels in the blood.
In children:
- Stiffness in arms and legs.
In adults:
- Increased intracranial pressure (which causes symptoms such as severe headache, vision problems, or vomiting).
- Rash.
- Itching.
- Hives on the skin with itching.
- Redness, itching, or pain at the injection site.
Formation of antibodies against the injected growth hormone, although this does not seem to affect the action of the growth hormone.
The skin around the injection site may become rough and irregular, but this should not occur if the injection is given at a different site each time.
The preservative metacresol may rarely cause muscle inflammation near the injection site as an adverse effect. If your doctor confirms that you have developed this adverse effect, you should use Genotonorm without metacresol.
There have been rare cases of sudden death in patients with Prader-Willi syndrome. However, no relationship has been established between these cases and treatment with this medicine.
If you experience discomfort or pain in the hip or knee while receiving treatment with Genotonorm, your doctor may consider the possibility that you suffer from slipped capital femoral epiphysis or Legg-Calvé-Perthes disease.
Other possible side effects related to growth hormone treatment are as follows.
You (or your child) may experience increased blood sugar levels or decreased thyroid hormone levels. Your doctor may perform tests to determine this and, if necessary, prescribe the appropriate treatment. Occasionally, pancreatitis has been reported in patients treated with growth hormone.
Reporting of Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaRAM.es.
By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Genotonorm Kabipen
Keep out of sight and reach of children.
Do not use this medicine after the expiration date stated on the packaging as MM/YYYY. The expiration date is the last day of the month indicated.
Before reconstitution
Store in a refrigerator (2°C-8°C). Keep the dual-chamber vial in the outer packaging to protect it from light.
Before opening, the medicine can be stored outside the refrigerator for a maximum of 1 month at a temperature not exceeding 25°C, but after this period, it must be discarded.
After reconstitution
Store in a refrigerator (2°C-8°C) for a maximum of 28 days. Do not freeze. Keep the pre-loaded GoQuick device in the outer packaging or the dual-chamber vial in the injection device case to protect it from light.
Do not use this medicine if you notice particles or if the solution is cloudy.
Do not freeze or expose this medicine to ice. If it freezes, do not use it.
Never throw away needles or empty vials in the regular trash. When you have finished using the needle, you must dispose of it carefully in a special container for needles, so that no one can use it or prick themselves.
Medicines should not be thrown down the drain or into the trash. Deposit the packaging and medicines you no longer need at the pharmacy's SIGRE point. If in doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This way, you will help protect the environment.
6. Container Contents and Additional Information
Composition of Genotonorm Kabipen
- The active ingredient is somatropin*.
- A vial contains 5.3 mg or 12 mg of somatropin*.
- After reconstitution, the somatropin* concentration is 5.3 mg or 12 mg per ml.
- The other components of the powder are: glycine (E640), mannitol (E421), anhydrous sodium hydrogen phosphate (E339), and anhydrous disodium dihydrogen phosphate (E339) (see section 2 "Genotonorm Kabipen contains sodium").
- The ingredients of the solvent are: water for injectable preparations, mannitol (E421), and metacresol.
*Obtained from Escherichia colicells by recombinant DNA technology.
Appearance of Genotonorm Kabipen and Container Contents
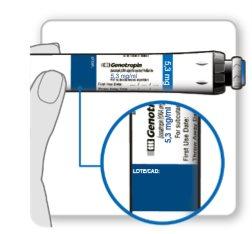
Powder and solvent for solution for injection, in a double-chamber vial containing the powder in one section and the solvent in the other (5.3 mg/ml or 12 mg/ml). The vial may be included in a preloaded device. Package sizes: 1 or 5 preloaded devices, or 1, 5, or 20 vials.
Only some package sizes may be marketed.
The powder is white and the solvent is transparent.
The vials must be used with a specific Genotonorm Kabipen injection device. The Genotonorm Kabipen vials have a color code and must be used with the Genotonorm Kabipen injection device that matches the color code to provide the correct dose: the Genotonorm Kabipen 5.3 mg vial (blue) must be used with the Genotonorm Kabipen 5.3 injection device (blue). The Genotonorm Kabipen 12 mg vial (purple) must be used with the Genotonorm Kabipen 12 injection device (purple).
The instructions for use of the device are included in its packaging. If you do not have an injection device, you can ask your doctor for one.
Marketing Authorization Holder
Pfizer, S.L.
Avda. de Europa 20 B
Parque Empresarial La Moraleja
28108 Alcobendas (Madrid), Spain.
Manufacturer
Pfizer Manufacturing Belgium NV
Rijksweg 12
2870 Puurs-Sint-Amands
Belgium
This medicinal product is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Genotropin: Austria, Denmark, Finland, Germany, Greece, Ireland, Italy, Netherlands, Portugal, Sweden, United Kingdom (Northern Ireland).
Genotonorm: Belgium, France, Luxembourg.
Genotonorm Kabipen: Spain.
Date of Last Revision of this Leaflet:May 2024
Detailed information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
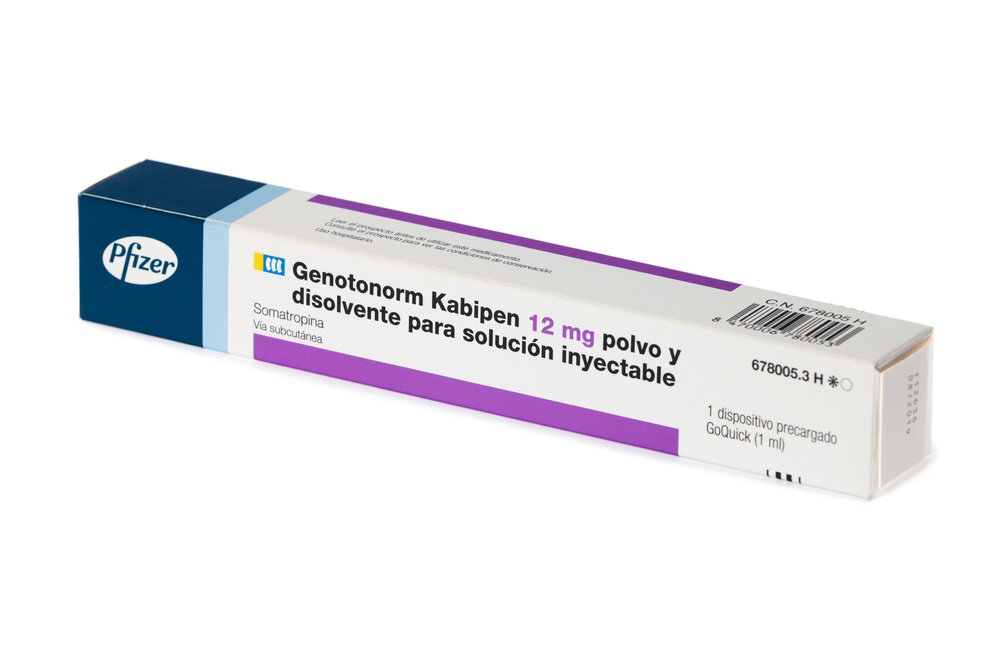
INSTRUCTIONS FOR USE OF GENOTONORM KABIPEN GOQUICK® Read these instructions completely before using the GoQuick device. If you have any doubts about the dose or treatment with Genotonorm, call your doctor or nurse. About GoQuick GoQuick is a preloaded, multidose, and disposable injection device that contains 5.3 mg of somatropin. The device can administer doses of 0.1 mg to 1.5 mg of the medication. Each click of the black wheel changes the dose by 0.05 mg. The vial of Genotonorm incorporated into the device is mixed only once, when you start using a new device. Never change the vial. When the device is empty, start using a new one. The device has a dose memory. The dose is adjusted each time you use a new device. Then, the device allows you to prepare the same dose established in each injection. This will prevent you from extracting a larger dose than established. Important Information
Storage and Disposal
Follow local health and safety regulations to dispose of (throw away) the device. Ask your doctor or nurse if you are not sure what to do. Parts of the GoQuick Device
The needles for the device are not includedwith the GoQuick device. You will need to purchase needles for devices up to 8 mm in length at the pharmacy.
Preparing and Using a New GoQuick Device |
(-:Step 1.)=100%(Step 1.:-)(-:Attach the Needle)=89%(Preparation
(-:Push the needle onto the cartridge holder tip.)=81%((-:Do not overtighten.)=100%( (-:Leave both needle covers on the needle.)=0%(<0} |
{0>Step 2.<}100{>Step 2.<0}{0>Mix the Genotropin<}0{>Choose the Injection Site<0}
<0} |
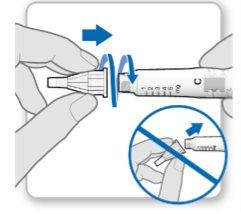
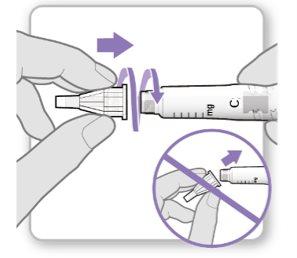
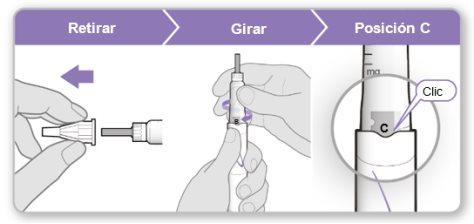
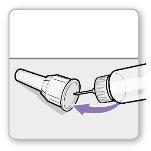
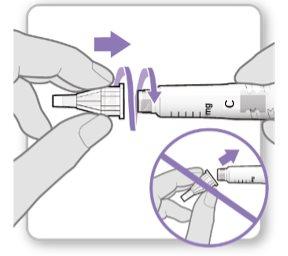
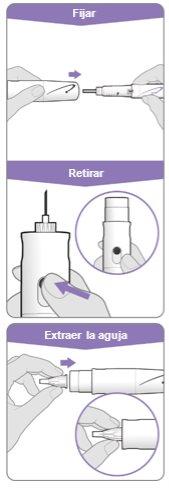
Step 3.Attach a New Needle
Note:Be careful not to attach the needle at an angle. This can cause leaks in the device.
<0} |
Step 4.Mix the Contents of the Genotonorm Vial
|
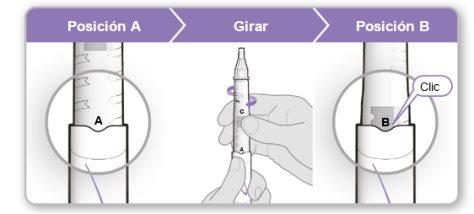
Step 5.Remove Air from the Device
Note:You should see an inner needle cover after removing the outer cover. If you do not see this, try attaching the needle again.
|
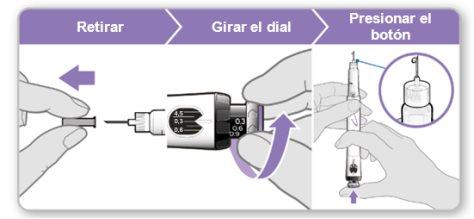
Step 6.Purge the Device
Purging removes the remaining air by pushing a small amount of liquid out of the device. The initial dose is 0.1 mg and is different from the dose prescribed by your doctor or nurse. Purge the device only the first time you use it.
Caution: Do nottouch the needle to avoid pricking yourself.
|
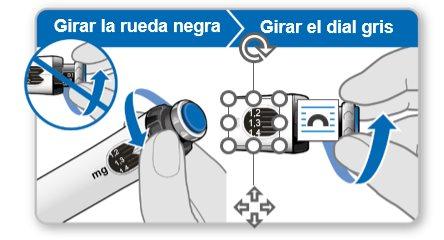
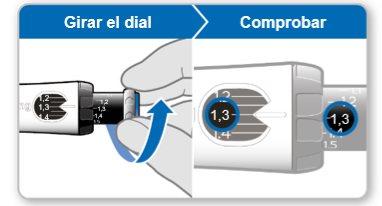
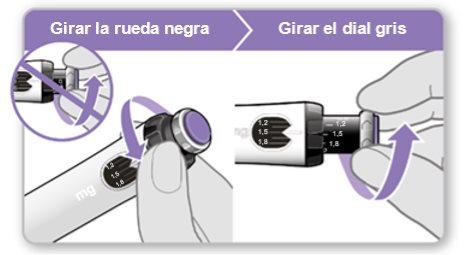
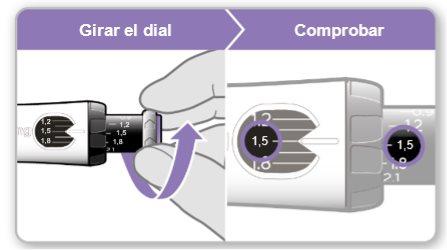
Step 7.Adjust and Prepare Your Dose
The first time you use the device, you will adjust the dose prescribed by your doctor or nurse. You do not need to adjust the dose again until you start using a new device or until your doctor or nurse tells you to.
Note:If you cannot rotate the black wheel, press the blue injection button until it stops clicking. Then try to adjust your dose again. Note that liquid will come out of the needle.
|
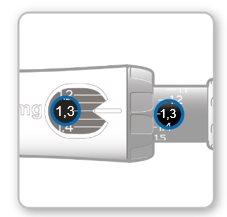
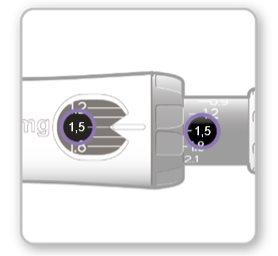
Step 8.Check Your Dose
Your dose should be alignedwith the white indicator.
|
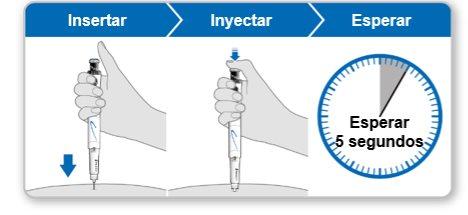
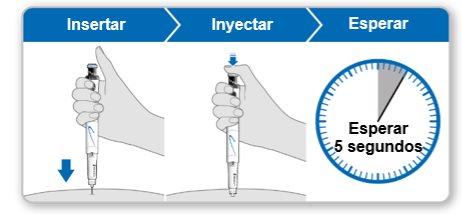
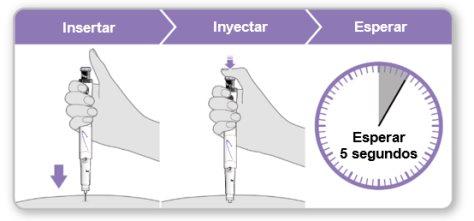
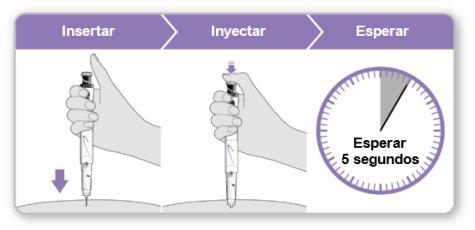
Step 9.Administer the Genotonorm Kabipen Injection
Note:If you see a drop of liquid at the injection site or on the tip of the needle, with the next injection, try pressing the blue injection button for a longer time before removing the needle from the skin. |
Step 10.Remove the Needle
Store the device in the refrigerator until the next injection. |
Usual Use (Daily) of the GoQuick Device | ||||||||||||||||||||||||||||||||||||||||
Step 1.Preparation
| ||||||||||||||||||||||||||||||||||||||||
Step 2.Choose the Injection Site
| ||||||||||||||||||||||||||||||||||||||||
Step 3.Attach a New Needle
| ||||||||||||||||||||||||||||||||||||||||
Step 4.Prepare Your Dose
|





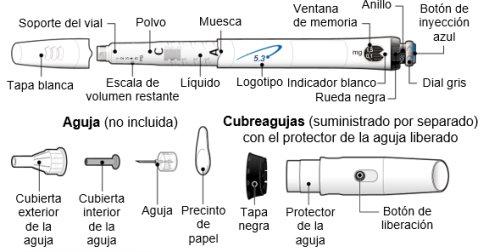
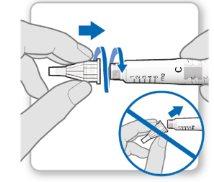
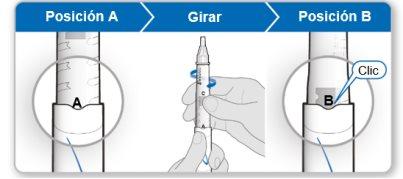
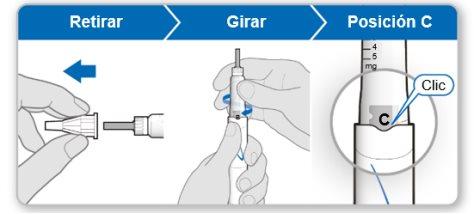
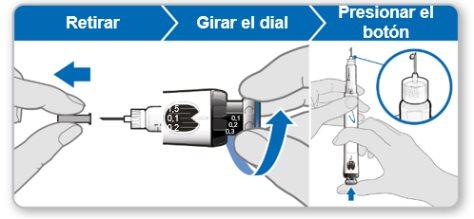
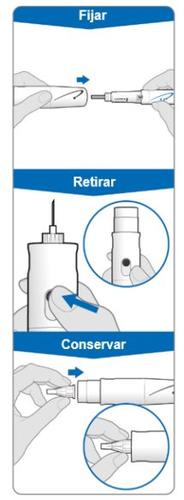 Using the needle shield (optional)
Using the needle shield (optional)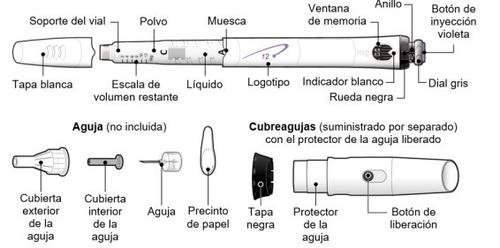
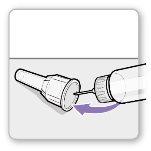
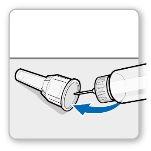
 Attach the needle shield:
Attach the needle shield:





