
VOLIBRIS 2.5 mg FILM-COATED TABLETS

How to use VOLIBRIS 2.5 mg FILM-COATED TABLETS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: information for the user
Volibris 2.5mg film-coated tablets
Volibris 5mg film-coated tablets
Volibris 10mg film-coated tablets
ambrisentan
Read the entire leaflet carefully before starting to take this medicine, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, consult your doctor, pharmacist, or nurse.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. See section 4.
Contents of the leaflet
- What is Volibris and what is it used for
- What you need to know before taking Volibris
- How to take Volibris
- Possible side effects
- Storage of Volibris
- Package contents and additional information
1. What is Volibris and what is it used for
Volibris contains the active substance ambrisentan. It belongs to a group of medicines called other antihypertensives (used to treat high blood pressure).
It is indicated for the treatment of pulmonary arterial hypertension (PAH) in adults, adolescents, and children aged 8 years and older. PAH is a condition where the blood pressure in the vessels (the pulmonary arteries) that carry blood from the heart to the lungs is elevated. In people with PAH, these arteries become narrower, so the heart has to work harder to pump blood to the lungs. This makes people feel tired, dizzy, and have difficulty breathing.
Volibris widens the pulmonary arteries, making it easier for the heart to pump blood through them. This reduces blood pressure and alleviates symptoms.
Volibris can also be used in combination with other medicines used to treat PAH.
2. What you need to know before taking Volibris
Do not take Volibris
- if you are allergicto ambrisentan, soy, or any of the other components of this medicine (listed in section 6).
- if you are pregnant, if you are planning to become pregnant, or if you may become pregnantbecause you are not using a reliable method of birth control (contraceptive). Please read the information in the "Pregnancy" section.
- if you are breastfeeding, read the information under the heading "Breastfeeding".
- if you have liver disease. Consult your doctor, who will decide if this medicine is suitable for you.
- if you have idiopathic pulmonary fibrosis(lung fibrosis of unknown cause).
Warnings and precautions
Consult your doctor before starting to take this medicine:
- if you have liver problems
- if you have anemia (reduced number of red blood cells)
- if you have swelling of the hands, ankles, or feet caused by fluid retention (peripheral edema)
- if you have a lung disease where the veins in the lungs are blocked (pulmonary veno-occlusive disease).
→ Your doctor will decideif Volibris is suitable for you.
You will need to have regular blood tests Before starting to take Volibris, and periodically while taking it, your doctor will perform blood tests to check:
- if you have anemia
- if your liver is functioning correctly.
→It is essential that you have these blood tests regularly while taking Volibris.
The signs that your liver may not be functioning properly include:
- loss of appetite
- nausea
- vomiting
- fever
- stomach pain (abdomen)
- yellowing of the skin or eyes (jaundice)
- darkening of the urine
- itching of the skin.
If you notice any of these circumstances:
→ Inform your doctor immediately.
Children
Do not give this medicine to children under 8 years of age, as the safety and efficacy in this age group are unknown.
Taking Volibris with other medicines
Tell your doctor or pharmacistif you are taking, have recently taken, or may need to take any other medicine.
If you start taking cyclosporin A(a medicine used after a transplant or to treat psoriasis), your doctor may need to adjust your Volibris dose.
If you are taking rifampicin(an antibiotic used to treat severe infections), your doctor will monitor you when you start taking Volibris.
If you are taking other medicines for PAH (iloprost, epoprostenol, sildenafil), your doctor may need to monitor you.
→ Inform your doctor or pharmacistif you are taking this medicine.
Pregnancy
Volibris may harm the fetus conceived before, during, or shortly after treatment.
→ If you may become pregnant, use a reliable contraceptive methodwhile taking Volibris. Consult your doctor about this.
→ Do not take Volibris if you are pregnant or planning to become pregnant.
→ If you become pregnant or think you may be pregnantwhile taking Volibris, consult your doctor immediately.
If you are a woman of childbearing age, your doctor will ask you to have a pregnancy testbefore starting to take Volibris and periodically while taking this medicine.
Breastfeeding
It is unknown if the active ingredient of Volibris can pass into breast milk.
→ Do not breastfeed while taking Volibris.Consult your doctor about this.
Fertility
If you are a man taking Volibris, this medicine may decrease your sperm count. Talk to your doctor if you have any questions or concerns about this.
Driving and using machines
Volibris may cause side effects such as low blood pressure, dizziness, fatigue (see section 4) that may affect your ability to drive and use machines. The symptoms of your disease may also reduce your ability to drive or use machines
→ Do not drive or use machines if you do not feel well.
Volibris contains lactose
The tablets of Volibris contain small amounts of a sugar called lactose. If your doctor has told you that you have an intolerance to certain sugars:
→ Consult your doctorbefore taking this medicine.
Volibris contains soy-derived lecithin
Do not use this medicine if you are allergic to soy (see section 2 "do not take Volibris").
The 5 mg and 10 mg tablets of Volibris contain an azoic colorant calledred allura AC aluminum lake (E129)
This may cause allergic reactions (see section 4).
Volibris contains sodium
This medicine contains less than 1 mmol of sodium (23 mg) per tablet; this is, essentially "sodium-free".
3. How to take Volibris
Follow the instructions for administration of this medicine exactly as indicated by your doctoror pharmacist.If you are unsure, consult your doctor or pharmacist again.
How much Volibris to take
AdultsThe usual dose is one 5 mg tablet, once a day. Your doctor may decide to increase your dose to 10 mg, once a day.
If you are taking cyclosporin A, do not take more than one 5 mg tablet of Volibris, once a day.
Adolescents and children from 8 years to less than 18 years of age
Usual starting dose of Volibris | |
Weight of 35 kg or more | One 5mgtablet, once a day |
Weight of at least 20 kg and less than 35 kg | One 2.5mgtablet, once a day |
Your doctor may decide to increase your dose. It is essential that children attend their regular medical appointments, as their dose needs to be adjusted as they grow or gain weight.
If taken in combination with cyclosporin A, the dose of Volibris should be limited to 2.5 mg once a day in adolescents and children who weigh less than 50 kg or to 5 mg once a day if they weigh 50 kg or more.
How to take VolibrisIt is best to take the tablet at the same time every day. Swallow the tablet whole, with a glass of water; do not divide, crush, or chew the tablet. You can take Volibris with or without food.
How to remove the tablet from the blister pack (only for 5 mg and 10 mg tablets)
These tablets come in a special packaging to prevent children from accessing them.
- Separate a tablet: tear along the cutting lines to separate a "cell" from the strip.
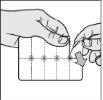
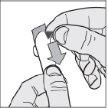
- Open the outer foil: starting from the colored corner, open and separate the foil along the cell.
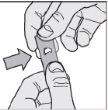
- Remove the tablet: gently push the tablet through the foil from one side.
The 2.5 mg tablets of Volibris are supplied in a bottle, not in a blister pack.
If you take more Volibris than you should
If you take too many tablets, you may be more likely to experience side effects, such as headache, flushing, dizziness, nausea (discomfort), or low blood pressure, which can cause a mild feeling of dizziness:
→ Ask your doctor or pharmacist for adviceif you take more tablets than prescribed.
If you forget to take Volibris
If you forget to take a dose of Volibris, take it as soon as you remember and then continue as before.
→ Do not take a double dose to make up for forgotten doses.
If you stop taking Volibris
Volibris is a treatment that you will need to continue taking to control your PAH.
→ Do not stop taking Volibris unless your doctor tells you to.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everyone will experience them.
Serious side effects
Tell your doctorif you experience any of the following:
Allergic reactions
This is a common side effect that may affect up to 1 in 10people. You may notice:
- a rash or itching and swelling (usually of the face, lips, tongue, or throat), which can cause difficulty breathing or swallowing.
Swelling (edema), especially of the ankles and feet
This is a very common side effect that may affect more than 1 in 10people.
Heart failure
This is because the heart does not pump enough blood. This is a common side effect that may affect up to 1 in 10people. The symptoms include:
- difficulty breathing
- extreme tiredness
- swelling in the ankles and legs.
Reduced number of red blood cells (anemia)
This is a very common side effect that may affect more than 1 in 10people. Sometimes this requires a blood transfusion. The symptoms include:
- tiredness and weakness
- difficulty breathing
- general discomfort.
Low blood pressure (hypotension)
This is a common side effect that may affect up to 1 in 10people. The symptoms include:
- dizziness
→ Tell your doctor immediatelyif you (or your child) experience these effects or if they occur suddenly after taking Volibris.
It is essential to have regular blood tests, to check if you have anemia and that your liver is functioning correctly. Also, make sure you have read the information in section 2about "the need for regular blood tests" and "signs that your liver may not be functioning properly".
Other side effects
Very common(may affect more than 1 in 10people)
- headache
- dizziness
- palpitations (rapid or irregular heartbeats)
- difficulty breathing that worsens shortly after starting to take Volibris
- runny nose or stuffy nose, congestion or pain in the nasal sinuses
- nausea
- diarrhea
- feeling of tiredness.
In combination with tadalafil (another medicine for PAH)
In addition to the above:
- flushing
- vomiting
- chest pain or discomfort.
Common(may affect up to 1 in 10people)
- blurred vision or other changes in vision
- fainting
- abnormal results in blood tests for liver function
- increased nasal secretion
- constipation
- stomach pain (abdomen)
- chest pain or discomfort
- flushing
- vomiting
- feeling of weakness
- nosebleeds
- skin rash.
In combination with tadalafil
In addition to the above, except for abnormal results in blood tests for liver function:
- ringing in the ears (tinnitus).
Uncommon(may affect up to 1 in 100people)
- liver damage
- inflammation of the liver caused by the body's immune system (autoimmune hepatitis).
In combination with tadalafil
- sudden loss of hearing.
Side effects in children and adolescents
These are expected to be similar to those listed above for adults.
Reporting side effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if they are not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Volibris
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging after EXP.
The expiry date is the last day of the month indicated.
This medicine does not require special storage conditions.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Volibris
The active ingredient is ambrisentan.
Each film-coated tablet contains 2.5 mg, 5 mg or 10 mg of ambrisentan.
For the 2.5mg tablets:
The other ingredients are: lactose monohydrate, microcrystalline cellulose, sodium croscarmellose, magnesium stearate, polyvinyl alcohol, talc, titanium dioxide (E171), macrogol and soy lecithin (E322).
For the 5mg and 10mg tablets:
The other ingredients are: lactose monohydrate, microcrystalline cellulose, sodium croscarmellose, magnesium stearate, polyvinyl alcohol, talc, titanium dioxide (E171), macrogol, soy lecithin (E322) and red allura AC aluminum lacquer (E129).
Appearance and Packaging of the Product
Volibris 2.5 mg film-coated tablets (tablet) are white, round, 7 mm, convex, engraved with “GS” on one side and “K11” on the other side.
Volibris 5 mg film-coated tablets (tablet) are pale pink, square, 6.6 mm, convex, engraved with "GS" on one side and "K2C" on the other side.
Volibris 10 mg film-coated tablets (tablet) are dark pink, oval, 9.8 mm x 4.9 mm, convex, engraved with "GS" on one side and "KE3" on the other side.
Volibris is supplied as 2.5 mg film-coated tablets in a bottle. Each bottle contains 30 tablets.
Volibris is supplied as 5 mg and 10 mg film-coated tablets in single-dose blisters, in packs of 10 × 1 or 30 × 1 tablets.
Not all pack sizes may be marketed.
Marketing Authorization Holder
GlaxoSmithKline (Ireland) Limited
12 Riverwalk
Citywest Business Campus
Dublin 24
Ireland
Manufacturer
GlaxoSmithKline Trading Services Limited
12 Riverwalk
Citywest Business Campus
Dublin 24
Ireland
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
Belgium GlaxoSmithKline Pharmaceuticals s.a./n.v. Tel: + 32 (0) 10 85 52 00 | Lithuania GlaxoSmithKline (Ireland) Limited Tel: + 370 80000334 |
| Luxembourg GlaxoSmithKline Pharmaceuticals s.a./n.v. Belgium Tel: + 32 (0) 10 85 52 00 |
Czech Republic GlaxoSmithKline s.r.o. Tel: + 420 222 001 111 | Hungary GlaxoSmithKline (Ireland) Limited Tel.: + 36 80088309 |
Denmark GlaxoSmithKline Pharma A/S Tlf: + 45 36 35 91 00 | Malta GlaxoSmithKline (Malta) Limited Tel: + 356 80065004 |
Germany GlaxoSmithKline GmbH & Co. KG Tel.: + 49 (0)89 36044 8701 | Netherlands GlaxoSmithKline BV Tel: + 31 (0) 33 2081100 |
Estonia GlaxoSmithKline (Ireland) Limited Tel: + 372 8002640 | Norway GlaxoSmithKline AS Tlf: + 47 22 70 20 00 |
Greece GlaxoSmithKline Μονοπρ?σωπη A.E.B.E. Tel: + 30 210 68 82 100 | Austria GlaxoSmithKline Pharma GmbH Tel: + 43 (0)1 97075 0 |
Spain GlaxoSmithKline, S.A. Tel: + 34 900 202 700 | Poland GSK Services Sp. z o.o. Tel.: + 48 (0)22 576 9000 |
France Laboratoire GlaxoSmithKline Tél: + 33 (0)1 39 17 84 44 | Portugal GlaxoSmithKline – Produtos Farmacêuticos, Lda. Tel: + 351 21 412 95 00 |
Croatia GlaxoSmithKline (Ireland) Limited Tel: + 385 800787089 | Romania GlaxoSmithKline (Ireland) Limited. Tel: + 40 800672524 |
Ireland GlaxoSmithKline (Ireland) Limited Tel: + 353 (0)1 4955000 | Slovenia GlaxoSmithKline (Ireland) Limited Tel: + 386 80688869 |
Iceland Vistor hf. Tel: + 354 535 7000 | Slovakia GlaxoSmithKline (Ireland) Limited Tel: + 421 800500589 |
Italy GlaxoSmithKline S.p.A. Tel: + 39 (0)45 7741 111 | Finland GlaxoSmithKline Oy Tel: + 358 (0)10 30 30 30 |
Cyprus GlaxoSmithKline (Ireland) Limited Tel: + 357 80070017 | Sweden GlaxoSmithKline AB Tel: + 46 (0)8 638 93 00 |
Latvia GlaxoSmithKline (Ireland) Limited Tel: + 371 80205045 | United Kingdom (Northern Ireland) GlaxoSmithKline (Ireland) Limited Tel: + 44 (0)800 221441 |
Date of the Last Revision of thisLeaflet:
Other Sources of Information
Detailed information on this medicinal product is available on the European Medicines Agency website: http://www.ema.europa.eu/. It also provides links to other websites on rare diseases and orphan medicines.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VOLIBRIS 2.5 mg FILM-COATED TABLETSDosage form: TABLET, 10 mgActive substance: ambrisentanManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: TABLET, 5 mgActive substance: ambrisentanManufacturer: Accord Healthcare S.L.U.Prescription requiredDosage form: TABLET, 10 mgActive substance: ambrisentanManufacturer: Alembic Pharmaceuticals Europe LimitedPrescription required
Online doctors for VOLIBRIS 2.5 mg FILM-COATED TABLETS
Discuss questions about VOLIBRIS 2.5 mg FILM-COATED TABLETS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions












