
TAMIFLU 6 mg/ml ORAL SUSPENSION POWDER

How to use TAMIFLU 6 mg/ml ORAL SUSPENSION POWDER
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet:information for the user
Tamiflu 6mg/ml powder for oral suspension
oseltamivir
Read all of this leaflet carefully before you start taking this medicine,because it contains important information for you.
?Keep this leaflet, you may need to read it again.
?If you have any further questions, ask your doctor or pharmacist.
?This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
?If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What is Tamiflu and what is it used for
- What you need to know before you take Tamiflu
- How to take Tamiflu
- Possible side effects
- Storing Tamiflu
- Contents of the pack and other information
1. What is Tamiflu and what is it used for
?Tamiflu is used in adults, adolescents, children, and infants (including newborn babies) to treat flu(influenza). It can be used when you have flu symptoms and it is known that the flu virus is present in the population.
?Tamiflu can also be prescribed to adults, adolescents, children, and infants over 1 year of age to prevent fluon a case-by-case basis, for example, if you have been in contact with someone who has the flu.
?Tamiflu may be prescribed to adults, adolescents, children, and infants (including newborn babies) as a preventive treatmentin exceptional circumstances, such as during a global flu epidemic (a flu pandemic) and when the seasonal flu vaccine cannot provide sufficient protection.
Tamiflu contains oseltamivir, which belongs to a group of medicines called neuraminidase inhibitors. These medicines prevent the flu virus from spreading inside the body. They help to relieve or prevent the symptoms of flu virus infection.
Flu is an infection caused by a virus. The signs of flu often include sudden fever (over 37.8°C), cough, runny or stuffy nose, headache, muscle pains, and extreme tiredness. These symptoms can also be caused by other infections. A true flu infection only occurs during annual outbreaks (epidemics), when flu viruses are widespread in the population. Outside of epidemic periods, flu-like symptoms are generally caused by another type of illness.
2. What you need to know before you take Tamiflu
Do not take Tamiflu
?if you are allergic(hypersensitive) to oseltamivir or any of the other ingredients of Tamiflu listed in section 6.
If this happens to you, consult your doctor. Do not take Tamiflu.
Warnings and precautions
Before taking Tamiflu, make sure your doctor is informed if:
?you are allergic to other medicines
?you have kidney disease. If so, your dose may need to be adjusted.
? if you have a severe illnessthat requires immediate hospitalization.
? if your immune systemis not working properly.
? if you have chronic heart diseaseor respiratory disease.
During treatment with Tamiflu, tell your doctor immediately
? if you notice changes in your behavior or mood (neuropsychiatric events), especially if they occur in children and adolescents. These can be signs of rare but serious side effects.
Tamiflu is not a flu vaccine
Tamiflu is not a vaccine: it is used to treat the infection or prevent the spread of the flu virus. A vaccine provides you with antibodies against the virus. Tamiflu does not affect the effectiveness of the flu vaccine, and your doctor may prescribe both.
Using Tamiflu with other medicines
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including those obtained without a prescription. This includes:
? chlorpropamide (used to treat diabetes)
? methotrexate (used to treat, for example, rheumatoid arthritis)
? phenylbutazone (used to treat pain and inflammation)
? probenecid (used to treat gout)
Pregnancy and breastfeeding
You should inform your doctor if you are pregnant, think you may be pregnant, or plan to become pregnant, so your doctor can decide if Tamiflu is suitable for you.
The effects on breastfed infants are unknown. You should inform your doctor if you are breastfeeding, so your doctor can decide if Tamiflu is suitable for you.
Consult your doctor or pharmacist before using this medicine.
Driving and using machines
Tamiflu has no effect on your ability to drive or use machines.
Important information about some of the ingredients of Tamiflu
Tamiflu contains sorbitol.
Sorbitol is a source of fructose. If your doctor has told you that you have an intolerance to some sugars or if you have been diagnosed with hereditary fructose intolerance (HFI), a rare genetic disorder in which a person cannot break down fructose, talk to your doctor before taking or receiving this medicine.
Sorbitol can cause gastrointestinal upset and a moderate laxative effect.
5 ml of oseltamivir suspension contains 0.9 g of sorbitol.
7.5 ml of oseltamivir suspension contains 1.3 g of sorbitol.
10 ml of oseltamivir suspension contains 1.7 g of sorbitol.
12.5 ml of oseltamivir suspension contains 2.1 g of sorbitol.
Tamiflu contains sodium benzoate.
Sodium benzoate (E211) may increase jaundice (yellowing of the skin and eyes) in newborns (up to 4 weeks of age).
5 ml of oseltamivir suspension contains 2.5 mg of sodium benzoate.
7.5 ml of oseltamivir suspension contains 3.75 mg of sodium benzoate.
10 ml of oseltamivir suspension contains 5.0 mg of sodium benzoate.
12.5 ml of oseltamivir suspension contains 6.25 mg of sodium benzoate.
This medicine contains less than 1 mmol of sodium (23 mg) per single dose (based on a maximum dose of 75 mg), i.e., it is essentially "sodium-free".
Tamiflu contains fructose
Before taking Tamiflu, make sure your doctor knows if you have hereditary fructose intolerance.
This medicine contains sorbitol, which is a type of fructose.
Sorbitol can have a moderate laxative effect.
3. How to take Tamiflu
Follow the instructions for taking Tamiflu exactly as your doctor has told you. If you are unsure, ask your doctor or pharmacist.
Always use the oral dispenser that is included in the box and has markings to indicate the dose in milliliters (ml).
Take Tamiflu as soon as possible, ideally within 2 days of starting to have flu symptoms.
Recommended doses
For the treatment of flu, take two doses daily. It is usually convenient to take one dose in the morning and one in the evening. It is important to complete the entire 5-day treatment, even if you start to feel better quickly.
For patients with a weakened immune system, treatment will continue for 10 days.
For the prevention of flu or after being in contact with an infected person, take one dose daily for 10 days. It is best to take this dose in the morning with breakfast.
In special situations, such as widespread flu or patients with a weakened immune system, treatment will continue for 6 or 12 weeks.
The recommended dose depends on the patient's body weight.You should use the amount of Tamiflu that your doctor has prescribed. Tamiflu oral suspension can be used in people who cannot swallow the capsules. See the instructions below for preparing and giving the dose.
Adults and adolescents 13 years and older
Body weight | Flu treatment:dose for 5 days | Flu treatment (in immunocompromised patients):dose for 10 days* | Flu prevention:dose for 10 days |
40 kg or more | 12.5 ml** twice daily | 12.5 ml** twice daily | 12.5 ml** once daily |
*For patients with a weakened immune system, treatment is for 10 days.
**12.5 ml can be prepared with a dose of 5 ml plus a dose of 7.5 ml
Children 1 to 12 years
Body weight | Flu treatment:dose for 5 days | Flu treatment (in immunocompromised patients):dose for 10 days* | Flu prevention:dose for 10 days |
10 to 15 kg | 5.0 ml twice daily | 5.0 ml twice daily | 5.0 ml once daily |
More than 15 kg and up to 23 kg | 7.5 ml twice daily | 7.5 ml twice daily | 7.5 ml once daily |
More than 23 kg and up to 40 kg | 10.0 ml twice daily | 10.0 ml twice daily | 10.0 ml once daily |
More than 40 kg | 12.5 ml** twice daily | 12.5 ml** twice daily | 12.5 ml** once daily |
*For children with a weakened immune system, treatment is for 10 days.
**12.5 ml is prepared with a dose of 5 ml plus a dose of 7.5 ml
Infants under 1 year (0 to 12 months)
Administration of Tamiflu to children under 1 year of age to prevent flu during a pandemic should be done based on medical judgment after considering the potential benefits and risks to the child.
A 3 ml oral dispenser (graduated with 0.1 ml markings) should be used for dosing in children under 1 year of age who require 1 to 3 ml of the Tamiflu oral suspension.
Body weight | Flu treatment:dose for 5 days | Flu treatment (in immunocompromised patients):dose for 10 days* | Flu prevention:dose for 10 days | Dispenser size to use |
3 kg | 1.5 ml twice daily | 1.5 ml twice daily | 1.5 ml once daily | 3 ml |
3.5 kg | 1.8 ml twice daily | 1.8 ml twice daily | 1.8 ml once daily | 3 ml |
4 kg | 2.0 ml twice daily | 2.0 ml twice daily | 2.0 ml once daily | 3 ml |
4.5 kg | 2.3 ml twice daily | 2.3 ml twice daily | 2.3 ml once daily | 3 ml |
5 kg | 2.5 ml twice daily | 2.5 ml twice daily | 2.5 ml once daily | 3 ml |
5.5 kg | 2.8 ml twice daily | 2.8 ml twice daily | 2.8 ml once daily | 3 ml |
6 kg | 3.0 ml twice daily | 3.0 ml twice daily | 3.0 ml once daily | 3 ml |
>6 to 7 kg | 3.5 ml twice daily | 3.5 ml twice daily | 3.5 ml once daily | 10 ml |
>7 to 8 kg | 4.0 ml twice daily | 4.0 ml twice daily | 4.0 ml once daily | 10 ml |
>8 to 9 kg | 4.5 ml twice daily | 4.5 ml twice daily | 4.5 ml once daily | 10 ml |
>9 to 10 kg | 5.0 ml twice daily | 5.0 ml twice daily | 5.0 ml once daily | 10 ml |
- For patients with a weakened immune system, treatment is for 10 days.
If you take more Tamiflu than you should
Stop taking Tamiflu and consult your doctor or pharmacist immediately.
In many cases of overdose, no side effects have been reported. When side effects have been reported, they were similar to those that occurred with normal doses and are included in section 4.
Overdose with Tamiflu has been reported more frequently in children than in adults and adolescents. Caution should be exercised when preparing Tamiflu liquid for children and when administering Tamiflu capsules or liquid to children.
If you forget to take Tamiflu
Do not take a double dose to make up for forgotten doses.
If you stop taking Tamiflu
No side effects occur when you stop taking Tamiflu. However, if you stop taking Tamiflu before your doctor told you to, your flu symptoms may return. Always complete the treatment that your doctor has prescribed.
If you have any further questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine can cause adverse effects, although not all people suffer from them. Many of these adverse effects can be caused by the flu.
Since the marketing of oseltamivir, the following serious adverse effects have been rarely reported:
? Anaphylactic and anaphylactoid reactions: severe allergic reactions, with swelling of the face and skin, itchy rash, low blood pressure, and difficulty breathing.
? Hepatic disorders (fulminant hepatitis, hepatic function disorder, and jaundice): yellowish skin and whites of the eyes, change in stool color, changes in behavior.
? Angioedema: sudden severe swelling of the skin mainly around the head and neck area, including eyes and tongue, with difficulty breathing.
? Stevens-Johnson syndrome and toxic epidermal necrolysis: complicated allergic reaction with possible life-threatening, severe inflammation of the outer and possibly inner skin, initially with fever, sore throat, and fatigue, skin rash, with blister formation, peeling, and large areas of peeled skin, possible breathing difficulties, and low blood pressure.
? Gastrointestinal hemorrhage: prolonged bleeding from the large intestine or vomiting blood
? Neuropsychiatric disorders, as described below.
If you notice any of these symptoms, seek medical help immediately.
The most frequently reported adverse effects (very frequent and frequent) for Tamiflu are feeling unwell or discomfort (nausea, vomiting), stomach pain, stomach discomfort, headache, and pain. These adverse effects usually occur after the first dose of the medicine and generally tend to disappear during treatment. The frequency with which these effects appear is reduced if the medicine is taken with food.
Rare but serious adverse effects: seek medical help immediately
(These can affect up to 1 in 1,000 people)
During treatment with Tamiflu, rare adverse effects have been reported, including
?seizures and delirium, including altered levels of consciousness
?confusion, abnormal behavior
?delirious disorders, hallucinations, agitation, anxiety, nightmares
These events were mainly reported in children and adolescents and often began suddenly and had a rapid resolution. In very rare cases, these events resulted in self-injury, some with a fatal outcome. These neuropsychiatric events have also been reported in patients with flu who were not taking Tamiflu.
? Patients, especially children and adolescents, should be closely monitored for changes in behavior described above.
If you notice any of these symptoms, especially in younger patients, seek medical help immediately.
Adults and adolescents 13 years and older
Very frequent adverse effects
(may affect more than 1 in 10 people)
? Headache
? Nausea
Frequent adverse effects
(may affect up to 1 in 10 people)
? Bronchitis
? Feverishness
? Cough
? Dizziness
? Fever
? Pain
? Pain in the limbs
? Runny nose
? Difficulty sleeping
? Sore throat
? Stomach pain
? Fatigue
? Feeling of fullness in the upper abdomen
? Upper respiratory tract infections (inflammation of the nose, throat, and sinuses)
? Stomach discomfort
? Vomiting
Uncommon adverse effects
(may affect up to 1 in 100 people)
? Allergic reactions
? Altered level of consciousness
? Seizure
? Heart rhythm disorders
? Liver function disorders from mild to severe
? Skin reactions (skin inflammation, red and itchy rash, scaly skin)
Rare adverse effects
(may affect up to 1 in 1,000 people)
? Thrombocytopenia (reduced number of platelets)
? Vision disorders
Children from 1 to 12 years
Very frequent adverse effects
(may affect more than 1 in 10 people)
? Cough
? Nasal congestion
? Vomiting
Frequent adverse effects
(may affect up to 1 in 10 people)
? Conjunctivitis (red and tearful eyes or pain in the eyes)
? Ear inflammation and other ear disorders
? Headache
? Nausea
? Runny nose
? Stomach pain
? Feeling of fullness in the upper abdomen
? Stomach discomfort
Uncommon adverse effects
(may affect up to 1 in 100 people)
? Skin inflammation
? Eardrum disorder (eardrum)
Infants under 1 year
The adverse effects reported in children from 0 to 12 months of age are mostly similar to the adverse effects reported in older children (from 1 year onwards). Additionally, diarrhea and diaper rash have been reported.
If you consider that any of the adverse effects you are experiencing is serious or if you notice any adverse effect not mentioned in this leaflet, inform your doctor or pharmacist. However,
?if you or your child are sick several times, or
?if the flu symptoms worsen or the fever continues
Tell your doctor as soon as possible.
Reporting of adverse effects
If you experience any type of adverse effect, consult your doctor, even if it is a possible adverse effect that does not appear in this leaflet. You can also report them directly through the national reporting system included in Appendix V.
By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Tamiflu
Keep this medicine out of the reach and sight of children.
Do not use this medicine after the expiration date that appears on the packaging and on the bottle after CAD. The expiration date is the last day of the month indicated.
Powder: Do not store above 30°C.
After reconstitution, the suspension can be stored at room temperature (below 25°C) for 10 days.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Package Contents and Additional Information
Tamiflu Composition
?The active ingredient is oseltamivir (6 mg/ml of oseltamivir after reconstitution)
?The other ingredients are: sorbitol (E420), sodium citrate dihydrate (E331[a]), xanthan gum (E415), sodium benzoate (E211), sodium saccharin (E954), titanium dioxide (E171), and tutti-frutti flavor (including maltodextrins (corn), propylene glycol, gum arabic E414, and natural flavoring substances (mainly banana, pineapple, and peach flavors).
Appearance of the Product and Package Contents
Oral solution powder.
The powder is a white to pale yellow granule or agglomerated granule.
Tamiflu 6 mg/ml oral suspension powder is available in bottles containing 13 g of powder to be mixed with 55 ml of water.
The packaging also includes 1 plastic measuring cup (55 ml), 1 plastic bottle adapter (to help insert the product into the dispenser), 1 plastic oral dispenser of 3 ml, and 1 plastic oral dispenser of 10 ml (to administer the correct amount of medicine orally). The oral dispenser has milliliter (ml) marks of the medicine (see figures in Instructions for Use).
For more details on how to prepare the oral suspension and how to measure and take the medicine, read the page on Instructions for Use”.
Marketing Authorization Holder and Manufacturer
Roche Registration GmbH
Emil-Barell-Strasse 1
79639 Grenzach-Wyhlen
Germany
Roche Pharma AG
Emil-Barell-Str. 1,
D-79639 Grenzach-Wyhlen
Germany
You can request more information about this medicine by contacting the local representative of the marketing authorization holder:
België/Belgique/Belgien N.V. Roche S.A. Tél/Tel: +32 (0) 2 525 82 11 | Lietuva UAB “Roche Lietuva” Tel: +370 5 2546799 |
???????? ??? ???????? ???? ???: +359 2 818 44 44 | Luxembourg/Luxemburg (See Belgium) |
Ceská republika Roche s. r. o. Tel: +420 - 2 20382111 | Magyarország Roche (Magyarország) Kft. Tel: +36 - 1 279 4500 |
Danmark Roche Pharmaceuticals A/S Tlf: +45 - 36 39 99 99 | Malta (See Ireland) |
Deutschland Roche Pharma AG Tel: +49 (0) 7624 140 | Nederland Roche Nederland B.V. Tel: +31 (0) 348 438050 |
Eesti Roche Eesti OÜ Tel: + 372 - 6 177 380 | Norge Roche Norge AS Tlf: +47 - 22 78 90 00 |
Ελλ?δα Roche (Hellas) A.E. Τηλ: +30 210 61 66 100 | Österreich Roche Austria GmbH Tel: +43 (0) 1 27739 |
España Roche Farma S.A. Tel: +34 - 91 324 81 00 | Polska Roche Polska Sp.z o.o. Tel: +48 - 22 345 18 88 |
France Roche Tél: +33 (0) 1 47 61 40 00 | Portugal Roche Farmacêutica Química, Lda Tel: +351 - 21 425 70 00 |
Hrvatska Roche d.o.o Tel: +385 1 4722 333 | România Roche România S.R.L. Tel: +40 21 206 47 01 |
Ireland Roche Products (Ireland) Ltd. Tel: +353 (0) 1 469 0700 | Slovenija Roche farmacevtska družba d.o.o. Tel: +386 - 1 360 26 00 |
Ísland Roche Pharmaceuticals A/S c/o Icepharma hf Sími: +354 540 8000 | Slovenská republika Roche Slovensko, s.r.o. Tel: +421 - 2 52638201 |
Italia Roche S.p.A. Tel: +39 - 039 2471 | Suomi/Finland Roche Oy Puh/Tel: +358 (0) 10 554 500 |
K?προς Γ.Α.Σταμ?της & Σια Λτδ. Τηλ: +357 - 22 76 62 76 | Sverige Roche AB Tel: +46 (0) 8 726 1200 |
Latvija Roche Latvija SIA Tel: +371 - 6 7039831 | United Kingdom (Northern Ireland) Roche Products (Ireland) Ltd. Tel: +44 (0) 1707 366000 |
Date of the last revision of thisleaflet:
Detailed information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu/.
Instructions for Use
There are two steps to take the oral suspension.
Step 1 Prepare a new bottle of the medicine
It is possible that your pharmacist has prepared the medicine when you pick up your prescription. If not, you can prepare it yourself easily. See the first set of instructions. You only need to do this once, when you start treatment.
Step 2 Measure and give the correct dose
Shake the suspension well and extract the recommended dose with the dispenser. See the second set of instructions. You will need to do this every time you need to take a dose.
Step 1 Prepare a new bottle of the medicine
You will need:
? The bottle containing the Tamiflu powder (in the medicine packaging)
? The bottle cap (in the medicine packaging)
? A plastic measuring cup (in the medicine packaging)
? The plastic bottle adapter (in the medicine packaging)
? Water
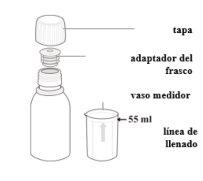
? Tap the bottle to loosen the powder
Gently tap the closed bottle several times to loosen the powder
? Use the measuring cup to measure 55ml of water
The measuring cup that comes with the packaging has a marked line to show you the exact amount.
Fill it with water up to the indicated level.
? Add the water, close, and shakePour all the water from the measuring cup into the bottle, over the powder
You must always use 55 ml of water, regardless of the dose you need.
Put the cap on the bottle. Shake the bottle well for 15 seconds.
? Press the adapter
Open the bottle and press the bottle adapter firmly into the neck of the bottle.
? Close the bottle again
Screw the cap tightly onto the top of the bottle, which now includes the adapter. This will ensure that the adapter fits into the bottle in the correct position.
You now have a prepared bottle of Tamiflu oral suspension ready to measure and administer a dose. You will not need to prepare it again until you start a new bottle.
Step 2: Measure and give the correct dose
You will need:
? A prepared Tamiflu oral suspension bottle
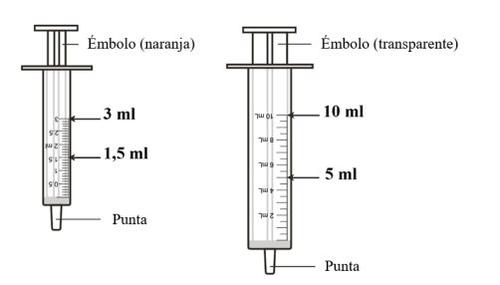
? Depending on the dose needed, you will need a 3ml oral dispenser (orange plunger, 0.1ml graduation) or a 10ml oral dispenser (transparent plunger, 0.5ml graduation) included in the packaging.
? For doses of 1.0 ml to 3.0 ml, the 3 ml oral dispenser should be used. For doses above 3.0 ml up to 10 ml, the 10 ml oral dispenser should be used.
Always use the oral dispenser provided in the packaging to measure the correct dose.
? Shake the bottle
Make sure the bottle is well closed and then shake the Tamiflu oral suspension bottle.
Always shake well before use.
? Prepare the oral dispenser
Depending on the dose needed, use the 3 ml oral dispenser (orange plunger) or the 10 ml oral dispenser (transparent plunger) that comes with the packaging.
Pushthe plunger all the way down towards the tip of the dispenser.
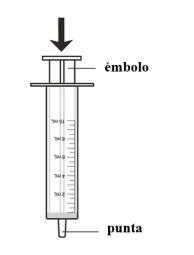
? Fill the dispenser with the correct dose
Unscrew the bottle cap.
Push the tip of the dispenser into the bottle adapter.
Turn the assembly(bottle and dispenser together) until the top isinverted.
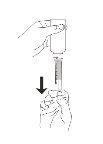
Slowly pull the plunger to draw the medicine into the dispenser
Stop at the graduation mark corresponding to the dose you need.
Turn the assembly back to the upright position.
Remove the dispenser from the bottle.
? Administer the medicine in the mouth
Insert the suspension directly into the mouth, pushing the dispenser plunger. Make sure the medicine has been swallowed. After taking the medicine, you can drink and eat some food.
? Close the bottle, keep in a safe place
Put the cap back on the bottle. Keep out of sight and reach of children.
Store the medicine below 25°C for up to 10 days. See section 5. Storage of Tamifluin this leaflet.
Immediately after administration, separate the parts of the dispenser and wash both under running water
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TAMIFLU 6 mg/ml ORAL SUSPENSION POWDERDosage form: CAPSULE, 30 mgActive substance: oseltamivirManufacturer: Actavis Group Ptc Ehf.Prescription requiredDosage form: CAPSULE, 75 mgActive substance: oseltamivirManufacturer: Actavis Group Ptc Ehf.Prescription requiredDosage form: CAPSULE, 30 mgActive substance: oseltamivirManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for TAMIFLU 6 mg/ml ORAL SUSPENSION POWDER
Discuss questions about TAMIFLU 6 mg/ml ORAL SUSPENSION POWDER, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















