
DERMATRANS 5 mg/24 H TRANSDERMAL PATCH

How to use DERMATRANS 5 mg/24 H TRANSDERMAL PATCH
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET:INFORMATION FOR THE USER
Dermatrans 5 mg/24 h, transdermal patch
Dermatrans 10 mg/24 h, transdermal patch
Dermatrans 15 mg/24 h, transdermal patch
Glyceryl trinitrate
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others, as it may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack:
- What is Dermatrans and what is it used for
- What you need to know before you use Dermatrans
- How to use Dermatrans
- Possible side effects
- Storing Dermatrans
- Contents of the pack and other information
1. What is Dermatrans and what is it used for
The Dermatrans patches contain the active ingredient glyceryl trinitrate, a vasodilator used in heart diseases, which belongs to a group of drugs called organic nitrates.
The Dermatrans patches are applied to the skin and then the active ingredient will pass continuously through your skin into your body.
Dermatrans is indicated for the prevention of angina attacks, either alone or in combination with other anti-anginal therapy.
Angina usually manifests as pain or pressure in the chest, although it can be felt in the neck or arm. The pain occurs when the heart is not sufficiently oxygenated. Dermatrans is not indicated for the treatment of acute attacks. For the treatment of acute attacks, you should use your usual sublingual or sublingual spray.
The Dermatrans patches are for external use only.
2. What you need to know before you use Dermatrans
Do not useDermatrans:
- if you are allergic to the active ingredient or any of the other ingredients of this medicine (listed in section 6).
- if you have recently had a shock associated with very low blood pressure;
- if you suffer from headaches (cephalalgia), vomiting, or convulsions associated with increased intracranial pressure, including those caused by head trauma;
- if you suffer from heart failure due to an obstruction, such as in the presence of aortic or mitral stenosis, or fibrotic thickening of the thin membrane surrounding the heart (constrictive pericarditis);
- if you are taking medicines for the treatment of erectile dysfunction (e.g., sildenafil or any other PDE-5 inhibitor). Nitrates should not be administered to patients treated with sildenafil or any other medication used to treat erectile dysfunction. Patients who are currently receiving nitrates should not take sildenafil or any other medication for the treatment of erectile dysfunction. The combination of a nitrate with sildenafil or any other PDE-5 inhibitor can cause a profound and sudden drop in blood pressure, which can lead to fainting, loss of consciousness, or even a heart attack (see also "Do not use Dermatrans");
- if you are taking medicines that contain riociguat, a soluble guanylate cyclase stimulator.
- if you have a severe drop in blood pressure (maximum arterial blood pressure of 90 mm Hg);
- if you suffer from a severe decrease in blood volume in your body due to significant blood loss or loss of other body fluids (severe hypovolemia);
- if you have severe anemia;
- if you have toxic fluid retention in the lungs (toxic pulmonary edema).
Warnings and precautions
Consult your doctor, pharmacist, or nurse before taking Dermatrans:
- if you stop treatment. The withdrawal of treatment with Dermatrans should be gradual, by substituting with decreasing doses of long-acting oral nitrates;
- if you are going to undergo a magnetic resonance imaging scan, electrical stimulation of your heart to restore a normal heart rhythm (defibrillation or cardioversion), and before a heat treatment (diathermy), you should remove the Dermatrans patch before undergoing these treatments.
- if you have recently had a heart attack (myocardial infarction) or if you develop rapidly worsening symptoms of heart failure (acute heart failure) such as difficulty breathing, feeling very tired, swelling of the legs. Your doctor may ask you to undergo laboratory tests to check your cardiovascular function.
- if you have a severe drop in blood pressure or if you suffer from collapse or shock while being treated with Dermatrans, the patch should be removed;
- if you experience chest pain (acute angina attacks) or if your heart is not getting enough blood flow and oxygen (unstable angina) or in case of a heart attack (myocardial infarction). Dermatrans should not be used as immediate treatment for these conditions.
- if you experience severe headache or abnormally low blood pressure (hypotension). This can occur if the initial dose is too high. It is advisable to gradually increase the dose until the optimal effect is achieved;
- if you are taking other nitrates or sublingual glyceryl trinitrate, as your body may develop resistance to the effects of these substances after repeated exposure (cross-tolerance);
- if you have or have had abnormally low blood pressure induced by glyceryl trinitrate. In this case, you may experience a low heart rate (paradoxical bradycardia) and increased angina;
- if you suffer from optic nerve disease (angle-closure glaucoma);
- if you have insufficient oxygenation of the blood (hypoxemia) due to severe anemia or lung disease or reduced blood supply to your heart (ischemic heart failure).
- Patient with these medical conditions often suffer from ventilation/perfusion imbalance, which is an index of respiratory function. In these patients, glyceryl trinitrate could worsen the ventilation/perfusion imbalance and cause further decrease in blood oxygenation;
- if angina is caused by thickening of the heart (hypertrophic cardiomyopathy). Nitrates can worsen this type of angina;
- if you experience an increase in the frequency of angina attacks during patch-free periods. It is possible that your doctor may want to evaluate the possibility of additional anti-anginal therapy.
- If you experience skin sensitization (itching, burning, inflammation), treatment should be discontinued and your doctor consulted.
Using other medicines
The simultaneous administration of medicines for the treatment of erectile dysfunction (e.g., sildenafil or any other PDE-5 inhibitor) enhances the blood pressure-lowering effects of nitrates and should therefore be avoided (see also "Do not use Dermatrans").
Concomitant treatment with riociguat, a soluble guanylate cyclase stimulator, should be avoided, as its use in combination with nitrates can cause hypotension (see also "Do not use Dermatrans").
Concomitant treatment with
- medicines used to lower high blood pressure, such as calcium antagonists, ACE inhibitors (for the treatment of congestive heart failure), beta-blockers (used to manage cardiac arrhythmias), diuretics (to increase water elimination from the body), and other antihypertensives,
- tricyclic antidepressants (medicines for the treatment of depressive disorders),
- neuroleptics (medicines used to treat psychosis), and
- major tranquilizers (sedatives),
- as well as alcohol consumption and the combination with amifostine (a cytoprotective medicine in chemotherapy and radiotherapy) and
- acetylsalicylic acid (an NSAID),
may enhance the blood pressure-lowering effects of Dermatrans.
Concomitant treatment with dihydroergotamine may reduce the effect of Dermatrans.
Non-steroidal anti-inflammatory drugs, except for acetylsalicylic acid, may decrease the therapeutic response to Dermatrans.
Tell your doctor or pharmacist if you are using, have recently used, or might use other medicines.
Fertility, pregnancy, and breastfeeding
Dermatrans should not be used during pregnancy, especially during the first three months, unless your doctor advises you to do so.
Due to limited information on the presence of glyceryl trinitrate in breast milk, the risk to the baby cannot be excluded. Your doctor will decide whether to discontinue breastfeeding or the use of Dermatrans.
No data are available on the effect of Dermatrans on human fertility.
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine.
Driving and using machines
Especially at the start of treatment or in case of dose adjustment, Dermatrans may affect your ability to drive or use machines, as it may reduce your reaction time or rarely cause hypotension when standing up and dizziness, as well as exceptional cases of fainting after an overdose.
If you experience these effects, do not drive or use machines.
3. How to use Dermatrans
Follow exactly the administration instructions of this medicine indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
The recommended dose is one Dermatrans patch once a day. You should apply the patch to your skin carefully and keep it on for 12-16 hours, then remove the patch and leave a patch-free period for the remaining 8-12 hours. You should change your Dermatrans patch according to the instructions given by your doctor. Your doctor will tell you how long to keep the patch on your skin and the duration of the patch-free interval.
Use in children and adolescents
Dermatrans should not be used in children and adolescents under 18 years of age.
How long to use Dermatrans
Treatment with Dermatrans can be continued for several years, however, your doctor should see you periodically to decide whether to continue treatment or change the therapeutic regimen.
How to apply the patch
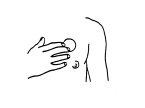 You should apply the patch to clean, dry skin, avoiding areas with cuts, spots, or imperfections, or areas where you have just applied cream, a moisturizing product, or talcum powder. It is recommended to apply the Dermatrans transdermal patches to the skin of the chest (see Figure 1) or the upper outer arm, in areas without redness or irritation, and change the application sites. The suitable area can be shaved if necessary. Areas that form folds or are subject to friction during movement should be avoided.
You should apply the patch to clean, dry skin, avoiding areas with cuts, spots, or imperfections, or areas where you have just applied cream, a moisturizing product, or talcum powder. It is recommended to apply the Dermatrans transdermal patches to the skin of the chest (see Figure 1) or the upper outer arm, in areas without redness or irritation, and change the application sites. The suitable area can be shaved if necessary. Areas that form folds or are subject to friction during movement should be avoided.
Figure 1
Do not apply two consecutive patches to the same place.
Once the Dermatrans patch is removed from the pouch, it should be applied immediately to the skin, as follows:
(I) Open the pouch at the notch.
Do not use scissors (see Figure 2).
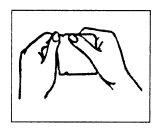
Figure 2
(II) Hold the patch with your thumb and index finger on the tab that will be removed
(see Figure 3).
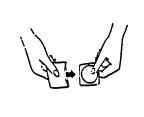 Figure 3
Figure 3
(III) Remove the protective liner with the other hand
(see Figure 4). Do not touch the adhesive side of the patch, as it will not stick well.
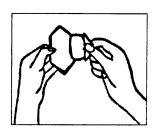
Figure 4
(IV) Apply the open part of the patch to the skin and remove the remaining part of the protective liner. Press firmly for about 10 seconds on the entire surface of the patch. Pass your fingers over the edges of the patch again to ensure it is firmly stuck.
Wash your hands before and after applying Dermatrans.
To remove a patch, simply peel it off from the edge and gently pull the patch until it comes off. After use, fold the patch in half, with the adhesive side inward, and throw it away in a trash can where children cannot reach it.
What to do if the patch falls off
If Dermatrans is applied correctly, it is very unlikely that the patch will fall off. However, if the patch falls off, replace it with a new one and follow the usual periodic schedule with the next patch.
If you use more Dermatrans than you should
If you apply high doses of glyceryl trinitrate, you may experience a severe drop in blood pressure, increased heart rate, or collapse and fainting, as well as alteration in hemoglobin (methemoglobinemia).
If you or someone else applies too many patches at the same time, remove the patches carefully and wash the underlying skin carefully to reduce absorption. In case you experience a drop in blood pressure or collapse, it is recommended that you elevate your legs or, if necessary, apply compression bandages.
If you forget to change the patch
If you forget to change the patch at the corresponding time, you should change it as soon as possible and then follow your original periodic schedule, applying the next patch.
If you stop treatment with Dermatrans
When you stop treatment with Dermatrans, you may experience a recurrence of angina attacks.
If you have any further questions on the use of this product, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, Dermatrans can cause side effects, although not everybody gets them.
The following side effects have been reported:
Very common side effects (affecting more than 1 in 10 people):
- Nausea.
- Vomiting.
Common side effects (affecting between 1 and 10 in 100 people)
- Headache.
Uncommon side effects (affecting between 1 and 10 in 1,000 people)
- Contact skin inflammation (contact dermatitis).
- Redness and skin irritation at the patch application site.
- Itching.
- Burning sensation.
Rare side effects (affecting between 1 and 10 in 10,000 people)
- Increased heart rate (tachycardia).
- Decrease in blood pressure when standing up (orthostatic hypotension) that can be described as transient episodes of dizziness.
- Redness.
- Increased heart rate as an analytical parameter.
Very rare side effects (affecting less than 1 in 10,000 people)
- Dizziness.
- Fainting (syncope).
Side effects of unknown frequency:
- Abnormal heartbeats (palpitations).
- Generalized skin rash (generalized rash).
Reporting of side effects
If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storing Dermatrans
Keep this medicine out of the sight and reach of children.
Do not store above 25°C. Dermatrans should be stored in its intact pouch.
Do not use this medicine after the expiry date which is stated on the carton and on the pouches. The expiry date is the last day of the month stated.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Container Content and Additional Information
Composition of Dermatrans
The Dermatrans patches contain the active ingredient glyceryl trinitrate and are available in three concentrations:
Dermatrans 5 mg/24 h: contains 15.70 mg of the active ingredient glyceryl trinitrate and releases approximately 5 mg of glyceryl trinitrate per day (0.2 mg/h); the patch release area is 6.38 cm2. The identification code printed on the foil is NR5.
Dermatrans 10 mg/24 h: contains 31.37 mg of the active ingredient glyceryl trinitrate and releases approximately 10 mg of glyceryl trinitrate per day (0.4 mg/h); the patch release area is 12.75 cm2. The identification code printed on the foil is NR10.
Dermatrans 15 mg/24 h: contains 47.04 mg of the active ingredient glyceryl trinitrate and releases approximately 15 mg of glyceryl trinitrate per day (0.6 mg/h); the patch release area is 19.12 cm2. The identification code printed on the foil is NR15.
The other ingredients are an adhesive substance (acrylate-vinyl acetate copolymer), an adhesion facilitator (hydroabietyl phthalate), and a cross-linking agent (butyl titanate polymer), which have been extended together with the active ingredient in a film (lacquered polypropylene film). The adhesive layer is covered by a two-sided, siliconized, and aluminum-coated protective film, which is removed before use.
Appearance of the Product and Container Content
Dermatrans are transdermal adhesive patches. Each patch is individually sealed in a protective envelope.
Package sizes: 15 and 30 patches. Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing authorization holder:
CASEN RECORDATI S.L.
Autovía de Logroño, km. 13,300 UTEBO
50180 (ZARAGOZA)
Manufacturer:
ROTTAPHARM LTD.
Damaastown, Industrial Park
15 Mulhuddart, County Dublin, Ireland
or
LTS Lohmann Therapie Systeme AG
Lohmannstraße 2
56626 Andernach
Germany
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
CountryName
Ireland Dermatrans
Italy Dermatrans
Spain Dermatrans
Date of the last revision of this leaflet: December 2022.
Detailed and updated information on this medicinal product is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price7.71 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to DERMATRANS 5 mg/24 H TRANSDERMAL PATCHDosage form: TRANSDERMAL PATCH, 37.4 mg nitroglycerinActive substance: glyceryl trinitrateManufacturer: Merus Labs Luxco Ii S.À.R.L.Prescription requiredDosage form: TRANSDERMAL PATCH, 18.7 mg nitroglycerinActive substance: glyceryl trinitrateManufacturer: Merus Labs Luxco Ii S.À.R.L.Prescription requiredDosage form: TRANSDERMAL PATCH, 31.37 mgActive substance: glyceryl trinitrateManufacturer: Casen Recordati S.L.Prescription required
Online doctors for DERMATRANS 5 mg/24 H TRANSDERMAL PATCH
Discuss questions about DERMATRANS 5 mg/24 H TRANSDERMAL PATCH, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











