
Nitromint

Ask a doctor about a prescription for Nitromint

How to use Nitromint
Leaflet attached to the packaging: information for the user
Nitromint
0.4 mg/dose, sublingual aerosol, solution
Glycerol trinitrate
You should carefully read the contents of the leaflet before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so that you can read it again if you need to.
- If you have any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Nitromint and what is it used for
- 2. Important information before using Nitromint
- 3. How to use Nitromint
- 4. Possible side effects
- 5. How to store Nitromint
- 6. Contents of the packaging and other information
1. What is Nitromint and what is it used for
Glycerol trinitrate, the active substance of Nitromint, is a vasodilator. Acting on the smooth muscles of blood vessels, glycerol trinitrate dilates peripheral veins and arteries, which reduces the preload and afterload on the heart, heart rate, oxygen demand, improves coronary circulation, and supplies oxygen to the ischemic heart muscle. Its action improves heart function and exercise tolerance.
Nitromint can be used in case of an acute angina pectoris attack (chest pain), as well as to prevent angina pectoris attacks by administering the medicine before expected physical exertion. It can be used as adjunctive treatment in acute cases of left ventricular failure.
Glycerol trinitrate administered to the sublingual mucosa is quickly absorbed, its action appears within 1 to 1.5 minutes.
2. Important information before using Nitromint
When not to use Nitromint
- If the patient is allergic to glycerol trinitrate, other nitrates, or any of the other ingredients of this medicine (listed in section 6) (if the patient has previously experienced facial, limb, lip, oral, tongue, larynx, and/or pharynx edema when using any nitrate-containing preparation).
- If the patient has angina pectoris caused by hypertrophic cardiomyopathy with outflow obstruction (abnormal heart enlargement).
- If the patient is using certain medicines for erectile dysfunction (medicines containing sildenafil, vardenafil, and tadalafil as active substances), as concurrent use of these medicines may lead to severe, life-threatening hypotension.
- If the patient has a disease that may lead to increased intracranial pressure (e.g., head injury, stroke).
- If the patient is using a medicine containing riociguat used to treat high blood pressure in the pulmonary arteries (pulmonary hypertension), as concurrent use may lead to a decrease in blood pressure.
Warnings and precautions
Before starting to use Nitromint, you should discuss it with your doctor or pharmacist.
Special caution and close medical supervision may be necessary:
- in case of shock (severe circulatory failure) or fainting (syncope),
- in certain cases of heart valve defects (e.g., aortic or mitral stenosis),
- if the patient has cardiac tamponade (fluid accumulation in the sac surrounding the heart),
- if the patient has constrictive pericarditis (inflammation of the smooth membrane surrounding the heart),
- if the patient has had problems in the past due to a sudden drop in blood pressure related to a change in body position (sitting or standing),
- in case of significant hypotension (low blood pressure with systolic blood pressure below 90 mmHg),
- if the patient has a disease in which the heart is unable to supply enough oxygenated blood to the rest of the body's tissues (cardiogenic shock),
- if the patient has a disease of the blood vessels supplying blood to the brain (cerebrovascular disease),
- if the patient has lung disease or heart disease associated with lung disease,
- if the patient has recently had a heart attack (myocardial infarction),
- if the patient has migraine,
- if the patient has glaucoma with closed-angle glaucoma (a type of increased intraocular pressure),
- if the patient has severe anemia (reduced red blood cell count or reduced hemoglobin level in the blood),
- if the patient has liver disease, alcoholic disease, epilepsy, brain injury, or other central nervous system disease (the aerosol contains a small amount of alcohol and may be harmful to patients with these disorders),
- if the patient is elderly, as the risk of hypotension or fainting is greater.
If symptoms of heart failure (breathing difficulties, leg swelling) worsen during treatment, you should consult your doctor immediately.
You should immediately call your doctor if you have coronary artery disease and chest pain occurs more frequently or in unusual situations, lasts longer, and does not respond to typical treatment.
Tolerance to this medicine or cross-tolerance to other nitrates may develop, i.e., the medicine may lose its effectiveness.
If symptoms do not disappear after administering a total of 3 doses (sprays), you should call the emergency services.
Lack of effect may indicate early myocardial infarction. In this case, you should call the emergency services.
The product is flammable, explosive, and the container should not be thrown into the fire, even when empty.
YOU MUST NOT use the aerosol near an open flame or while smoking.
Glycerol trinitrate increases the excretion of certain organic substances, such as catecholamines and VMA (vanillylmandelic acid), in the urine. Before performing blood or urine tests, you should tell your doctor that you are using Nitromint.
Children
There is no data on the use of Nitromint in children.
Nitromint and other medicines
You should tell your doctor about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take.
NEVER use Nitromint
- with medicines containing sildenafil, vardenafil, and tadalafil for the treatment of erectile dysfunction (this may increase the blood pressure-lowering effect of Nitromint);
- with medicines containing riociguat used to treat high blood pressure in the pulmonary arteries (pulmonary hypertension), as this may cause a decrease in blood pressure.
Concomitant use with the following medicines is subject to the decision of the attending physician
- Medicines with blood pressure-lowering effects, such as vasodilators and other antihypertensive medicines, neuroleptics (used to treat mental illnesses), medicines used to treat depression (e.g., tricyclic antidepressants), sapropterin (used to treat phenylketonuria), and N-acetylcysteine (amino acid supplement). Each of these medicines may increase blood pressure, weakening the effect of Nitromint.
- Dihydroergotamine (used to treat migraine or to stimulate uterine contractions). Nitromint may enhance the effect of this medicine.
- Blood-thinning preparations containing heparin (the effectiveness of heparin may decrease).
- Medicines used to treat acute or chronic pain and inflammation (nonsteroidal anti-inflammatory drugs, except for acetylsalicylic acid). The effect of Nitromint may be weakened.
- Amifostine (used to treat cancer) may increase blood pressure, weakening the effect of Nitromint.
- Acetylsalicylic acid (may enhance the blood pressure-lowering effect of glycerol trinitrate; additive inhibition of platelet function has also been demonstrated).
- Disulfiram - there is no data on possible interaction between disulfiram and Nitromint containing a small amount of ethanol. Since such an interaction cannot be ruled out, this fact should be taken into account in case of concomitant use of both medicines.
Patient previously treated with organic nitrate medicines (e.g., isosorbide dinitrate, isosorbide mononitrate) may require higher doses of glycerol trinitrate.
If your doctor has prescribed sublingual tablets for use in case of an angina pectoris attack, you should not use the aerosol during the same attack.
Using Nitromint with alcohol
Consuming alcoholic beverages while using this medicine is strictly prohibited, as some side effects may occur more severely than usual (see section 4).
- 4).
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, you should consult your doctor or pharmacist before using any medicine.
Pregnancy
Nitromint may be used in pregnant women only if the doctor considers that the benefits to the mother outweigh the potential risk to the fetus.
Breastfeeding
Considering the benefits of breastfeeding for the baby and the benefits of treatment for the mother, a decision should be made to either stop breastfeeding or stop treatment with Nitromint.
Driving and operating machines
Dizziness and fainting may occur during the initial phase of treatment. For this reason, you should avoid driving vehicles, operating machines, and performing activities with increased risk of accidents. Then, you should consult your doctor to determine if you can perform these activities.
Nitromint contains ethanol (alcohol) and propylene glycol
This medicine contains 42.65 mg of ethanol (alcohol) in each dose (1 spray).
The amount of alcohol in a dose of this medicine is equivalent to less than 2 mL of beer or 1 mL of wine.
A small amount of alcohol in this medicine will not cause noticeable effects.
This medicine contains 11.22 mg of propylene glycol in each dose (1 spray), which may cause irritation to the mucous membranes.
3. How to use Nitromint
This medicine should always be used according to the doctor's or pharmacist's recommendations. If you have any doubts, you should consult your doctor or pharmacist.
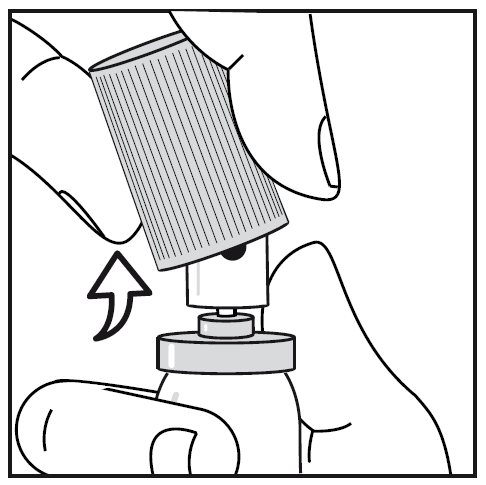
The cap can be easily removed from the container (see Figure 1 below).
Figure 1. Removing the cap from the container
The pump dispenser should be filled before the first use of the medicine by removing the protective cap and performing a few sprays into the air until the aerosol appears. It may be necessary to refill the pump dispenser if it has not been used for a long time.
Shaking the dispenser before use is not necessary.
If possible, you should sit down.
Remove the cap. Hold the container vertically with your index finger on the white dispenser button. Place the dispenser outlet near your mouth. For a moment, hold your breath to avoid inhaling the aerosol, then open your mouth and spray the dose of aerosol under your tongue by pressing the pump button and releasing the aerosol.
Immediately close your mouth, but DO NOT inhale the medicine.Hold your breath while using the medicine.
After using the medicine, you should put the cap back on the aerosol container.
You should always carry the medicine with you, as you may need to use it quickly.
Each use should be marked on the packaging.
You should always have a spare medicine, as it may run out.
It may be helpful to learn where the dispenser outlet is, so that administering the medicine at night does not cause difficulties.
Use as directed by your doctor.
Pressing the filling valve delivers one measured dose of aerosol (0.4 mg of glycerol trinitrate) from the container equipped with a mechanical pump.
The following doses are recommended:
in case of angina pectoris attacks, you should spray 1 dose (spray) under your tongue. If symptoms do not disappear, this dose (1 spray) can be repeated at 5-minute intervals, up to a total of 3 doses (aerosol sprays). If symptoms do not disappear after 3 doses (sprays), you should call the emergency services.
Lack of effect may indicate early myocardial infarction. In this case, you should call the emergency services.
In case of acute cardiogenic pulmonary edema, in patients who are not in a state of hypotension (i.e., with systolic blood pressure > 100 mmHg), you should spray 1 dose (spray) under your tongue. If symptoms do not disappear, this dose (1 spray) can be repeated at 5-10 minute intervals, while carefully monitoring the patient's clinical condition, including blood pressure. If symptoms persist, your doctor may change the medicine to intravenous or another vasodilator, depending on the clinical response.
To prevent angina pectoris attacks, you should use 1 dose (aerosol spray) before exertion that may trigger angina pectoris attacks in you.
Elderly patients
A drop in blood pressure and fainting may be a particular problem when using nitrates in elderly patients. When taking glycerol trinitrate sublingually, you should sit down, if possible.
Children and adolescents
Nitromint is not recommended for use in children.
Using a higher than recommended dose of Nitromint
You should immediately call your doctor if you have used a dose higher than recommendedand have symptoms of overdose (headache, hypotension, rapid heartbeat, dizziness, hot flashes, nausea, vomiting, diarrhea, shortness of breath, or rapid breathing).
If you have any further doubts about using this medicine, you should consult your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Very common(may affect more than 1 in 10 people)
- Headache
Common(may affect up to 1 in 10 people)
- Dizziness
- Drowsiness
- Rapid heartbeat
- Low blood pressure*
- Drop in blood pressure when standing or sitting*
- Weakness
Uncommon(may affect up to 1 in 100 people)
- Fainting
- Worsening of angina pectoris symptoms (worsening of chest pain)
- Slow heartbeat
- Cyanosis (blue or purple discoloration of the skin or mucous membranes due to low oxygen levels in the tissues just below the skin surface)
- Flushing
- Circulatory collapse (rarely with slow heart rate and loss of consciousness)
- Nausea
- Vomiting
Rare(may affect up to 1 in 10,000 people)
- Methemoglobinemia (a disorder characterized by the presence of a higher than normal concentration of methemoglobin in the blood. This can lead to a decrease in the ability of red blood cells to release oxygen to tissues).
- Restlessness
- Decreased oxygen supply to the brain
- Heartburn
- Unpleasant mouth odor
- Breathing difficulties
- Skin inflammation
- Rash
Headache is the most commonly reported adverse reaction to glycerol trinitrate, and it may occur at the beginning of treatment. This so-called "nitrate headache" usually disappears after a few days.
*Especially during the initiation of therapy and after dose increase.
Frequency not known(frequency cannot be estimated from the available data)
- Allergic reactions may occur.
- Palpitations (feeling of strong heartbeat)
- Administration of the medicine may cause mild, transient burning sensation in the mouth.
Reporting side effects
If you experience any side effects, including any side effects not listed in this leaflet, you should tell your doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C, 02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Nitromint
Store in a temperature below 25°C.
Store in the original packaging to protect from light.
The medicine should be stored out of sight and reach of children.
The product is flammable, explosive.
Storage and use near an open flame or while smoking are prohibited.
Do not use this medicine after the expiry date (EXP) stated on the container. The expiry date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. You should ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Nitromint contains
The active substance of Nitromint is glycerol trinitrate. Each dose contains 0.4 mg of glycerol trinitrate in the form of a 1% ethanol solution.
The other ingredients are ethanol, propylene glycol.
What Nitromint looks like and what the packaging contains
Almost colorless, clear, sediment-free solution with a weak characteristic odor.
The packaging contains 11 g (200 doses) in a metal aerosol container equipped with a mechanical pump and dispenser, closed with a protective cap, placed in a folded cardboard box with a Patient Information Leaflet attached.
Marketing authorization holder
PROTERAPIA Spółka z o.o.
ul. Komitetu Obrony Robotników 45D
02-146 Warsaw
Manufacturer
EGIS Pharmaceuticals PLC
9900 Körmend Mátyás király u.65
Hungary
To obtain more detailed information about this medicine, you should contact:
PROTERAPIA Spółka z o.o.
ul. Komitetu Obrony Robotników 45D
02-146 Warsaw
Phone: +48 22 417 92 00
Date of last revision of the leaflet:22.05.2023
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterEGIS Pharmaceuticals PLC
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to NitromintDosage form: Ointment, 20 mg/gActive substance: glyceryl trinitrateManufacturer: Chema-Elektromet Spółdzielnia PracyPrescription not requiredDosage form: Solution, 1 mg/mlActive substance: glyceryl trinitrateManufacturer: Aesica Pharmaceuticals GmbHPrescription not requiredDosage form: Tablets, 6.5 mgActive substance: glyceryl trinitratePrescription required
Alternatives to Nitromint in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Nitromint in Spain
Alternative to Nitromint in Ukraine
Online doctors for Nitromint
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Nitromint – subject to medical assessment and local rules.










