
AXALHIS 1 mg/ml NASAL SPRAY SOLUTION

How to use AXALHIS 1 mg/ml NASAL SPRAY SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
AXALHIS 1 mg/ml nasal spray solution
azelastine hydrochloride
Read this package leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others, as it may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet.
Contents of the package leaflet:
- What AXALHIS is and what it is used for
- What you need to know before you use AXALHIS
- How to use AXALHIS
- Possible side effects
- Storage of AXALHIS
- Contents of the pack and further information
1. What AXALHIS is and what it is used for
AXALHIS contains azelastine, which belongs to a group of medicines that prevent the effects of histamine (antihistamines) and other substances that the body produces as part of an allergic reaction, which cause symptoms of sneezing, runny nose, itching or nasal congestion. Azelastine also has an additional anti-inflammatory effect.
This medicine is used to treat the symptoms of seasonal allergic rhinitis and acute exacerbations (attacks) of perennial allergic rhinitis.
AXALHIS nasal spray solution is intended for use by adults, adolescents, and children over 6 years of age.
2. What you need to know before you use AXALHIS
Do not useAXALHIS
- if you are allergic to azelastine or any of the other ingredients of this medicine (listed in section 6).
This medicine must not be used in children under 6 years of age. The instructions for adolescents are the same as for adults (see also section 3 "Use in children and adolescents").
Warnings and precautions
The spray must be used with the head in an upright position.
Consult your doctor or pharmacist before you start using this medicine.
Children and adolescents
This product must not be used in children under 6 years of age.
.
Using AXALHIS with other medicines.
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines.
No specific interactions have been studied.

Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
There are no or limited data on the use of azelastine in pregnant women. Therefore, this medicine should only be used during pregnancy if recommended by a doctor.
Due to the lack of data, AXALHIS nasal spray solution must not be used during breastfeeding.
Driving and using machines
You may rarely experience fatigue, tiredness, exhaustion, dizziness, or weakness due to the same condition or when using the nasal spray. In these cases, do not drive or operate machinery.
Note that the consumption of alcohol or other medicines may increase these effects.
3. How to use AXALHIS
Follow exactly the administration instructions of this medicine given by your doctor or pharmacist. If you are in doubt, consult your doctor or pharmacist again.
Adults, adolescents, and children over 6 years of age
The recommended dose is one spray (0.14 ml) into each nostril, twice a day (0.56 mg of azelastine hydrochloride).
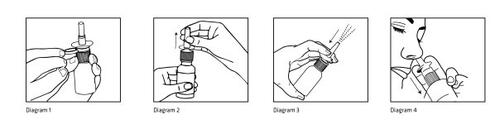
Instructions for the correct administration of the medicine
- Blow your nose
- Before the first use, remove the plastic cap from the nozzle (Diagram 1)
- Remove the protective cap (Diagram 2)
- Before the first use, press the pump several times until a uniform spray comes out (3-4 times) (Diagram 3)
- Spray once into each nostril keeping your head in an upright position. Do not tilt your head back(Diagram 4).
- Clean the pump nozzle and replace the protective cap
When the product has not been used for 6 days or more, reactivate the pump by pressing down and releasing a sufficient number of times until a fine mist comes out.
Use in children and adolescents
This medicine must not be used in children under 6 years of age due to the lack of safety and efficacy data.
If you use more AXALHISthan you should
If you have sprayed too much of this medicine, contact your doctor or pharmacist. With nasal administration, overdose reactions are not expected. Studies conducted in animals have revealed that toxic doses can produce symptoms in the central nervous system (excitement, tremors, convulsions). If this occurs in humans, symptomatic and supportive treatment should be initiated. If the overdose is recent, gastric lavage is recommended.
In case of overdose or accidental ingestion, consult your doctor or pharmacist or call the Toxicology Information Service, phone: 91 562 04 20, indicating the medicine and the amount ingested 
If you forget to use AXALHIS
Do not use a double dose to make up for forgotten doses.
If you forget to use your medicine, use it as soon as you remember and take the next dose 12 hours later, if necessary.
If you stop using AXALHIS
Treatment with azelastine should be done regularly until the symptoms are relieved.
If you stop treatment with azelastine nasal spray, the symptoms of your allergy may recur. Follow the instructions and do not exceed the recommended duration of use indicated by your doctor.
.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Common side effects (may affect up to 1 in 10 people): you may experience an unpleasant taste after administration (often due to incorrect application, for example, tilting the head too far back during administration), which may rarely cause nausea.
Uncommon side effects (may affect up to 1 in 100 people): nasal discomfort due to inflammation of the nasal tissue (itching, burning), sneezing, nasal bleeding.
Rare side effects (may affect up to 1 in 1,000 people): nausea
Very rare side effects (may affect up to 1 in 10,000 people): fatigue (tiredness, exhaustion), dizziness or weakness (may be caused by the condition itself), hypersensitivity reactions, rash, itching, urticaria (nettle rash).
If any of the side effects gets serious, or if you notice any side effects not listed in this package leaflet, please tell your doctor or pharmacist.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. You can also report side effects directly through the Spanish Medicines Monitoring System www.notificaram.es.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of AXALHIS
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date is the last day of the month stated.
Do not refrigerate or freeze.
Do not store above 25°C.
Do not use for more than 6 months after first opening.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help protect the environment.
6. Contents of the pack and further information
Composition of AXALHIS
 The active substance is azelastine hydrochloride 1 mg/ml
The active substance is azelastine hydrochloride 1 mg/ml- The other ingredients (excipients) are: hypromellose 2910, disodium edetate, citric acid, disodium phosphate dodecahydrate, sodium chloride, and purified water
Appearance of AXALHIS and contents of the pack
AXALHIS is a clear, colorless, and particle-free solution.
AXALHIS nasal spray solution is filled in a multidose plastic container (high-density polyethylene) equipped with a dosing pump. Each bottle contains 10 ml of solution.
Marketing authorisation holder
Zentiva, k.s.
U kabelovny 130,
Dolní Mecholupy,
102 37 Prague 10
Czech Republic
Manufacturer
SAG Manufacturing S.L.U.
Carretera N-I, Km 36
28750 San Agustín de Guadalix – Madrid
Spain
You can request more information about this medicine from the local representative of the marketing authorisation holder:
Zentiva Spain S.L.U.
Avenida de Europa, 19, Edificio 3, Planta 1.
28224 Pozuelo de Alarcón, Madrid
Date of last revision of this package leaflet: March 2023
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Healthcare Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AXALHIS 1 mg/ml NASAL SPRAY SOLUTIONDosage form: NASAL PRODUCT, 0.1 g azelastine hydrochloride/100 mlActive substance: azelastineManufacturer: Cooper Consumer Health B.V.Prescription requiredDosage form: NASAL PRODUCT, 1.5 mg/mlActive substance: azelastineManufacturer: Cooper Consumer Health B.V.Prescription requiredDosage form: NASAL PRODUCT, 1.5 mg/mlActive substance: azelastineManufacturer: Cooper Consumer Health B.V.Prescription required
Online doctors for AXALHIS 1 mg/ml NASAL SPRAY SOLUTION
Discuss questions about AXALHIS 1 mg/ml NASAL SPRAY SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











