

Azelamed

Ask a doctor about a prescription for Azelamed

How to use Azelamed
Package Leaflet: Information for the Patient
Azelamed, 1 mg/ml, Nasal Spray, Solution
Azelastine Hydrochloride
For use in adults, adolescents, and children over 6 years of age
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in the package leaflet for the patient or as directed by a doctor or pharmacist.
- This leaflet should be kept in case it needs to be read again.
- If advice or additional information is needed, a pharmacist should be consulted.
- If the patient experiences any side effects, including any possible side effects not listed in the leaflet, they should inform their doctor or pharmacist. See section 4.
- If there is no improvement after 2 days or the patient feels worse, they should contact their doctor.
Table of Contents of the Leaflet
- 1. What is Azelamed and what is it used for
- 2. Important information before using Azelamed
- 3. How to use Azelamed
- 4. Possible side effects
- 5. How to store Azelamed
- 6. Contents of the pack and other information
1. What is Azelamed and what is it used for
Azelamed contains azelastine, which belongs to a group of antihistamine medicines. Antihistamines work by preventing the action of histamine produced by the body as a result of an allergic reaction. Azelastine has been shown to reduce inflammation of the nasal mucosa. Azelamed is used to treat symptoms of hay fever (seasonal, allergic rhinitis) in adults, adolescents, and children over 6 years of age.
If there is no improvement after 2 days or the patient feels worse, they should contact their doctor.
2. Important information before using Azelamed
When not to use Azelamed
- if the patient is allergic to azelastine hydrochloride or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting to use Azelamed, the patient should discuss it with their doctor or pharmacist.
If the head is tilted too far back during administration, drowsiness and a bitter taste in the mouth may occur due to increased absorption.
Other medicines and Azelamed
The patient should tell their doctor or pharmacist about all medicines they are currently taking or have recently taken, as well as any medicines they plan to take.
No interactions with other medicines have been observed so far.
Azelamed can be used with other antihistamines and/or central nervous system depressants only after consulting a doctor.
Using Azelamed with alcohol
As a rule, the patient should avoid drinking alcoholic beverages while using any medicines.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a baby, they should consult their doctor or pharmacist before using this medicine.
Pregnancy
There is insufficient experience with the use of azelastine during pregnancy. Therefore, to avoid risk, Azelamed should not be used during the first three months of pregnancy.
In the second and third trimesters of pregnancy, Azelamed should only be used if absolutely necessary and under medical supervision.
Breastfeeding
The active substance of Azelamed passes into breast milk in small amounts. Therefore, Azelamed should not be used during breastfeeding.
Driving and using machines
In very rare cases, patients may experience symptoms such as fatigue, exhaustion, dizziness, or weakness, which may also be caused by the disease. In such cases, the ability to drive and use machines may be impaired.
Consuming alcohol or taking other medicines that affect alertness may exacerbate these symptoms.
3. How to use Azelamed
This medicine should always be used exactly as described in the package leaflet for the patient or as directed by a doctor or pharmacist. If in doubt, the patient should consult their doctor or pharmacist.
The recommended dose is 1 spray of Azelamed into each nostril, twice a day (morning and evening) (which corresponds to a dose of 0.56 mg of azelastine hydrochloride per day).
Children
There are no available appropriate studies on dosing and pharmacokinetics; therefore, Azelamed should not be used in children under 6 years of age.
Patient with liver or kidney impairment
There is insufficient experience with the treatment of Azelamed in patients with liver or kidney impairment.
Method of administration
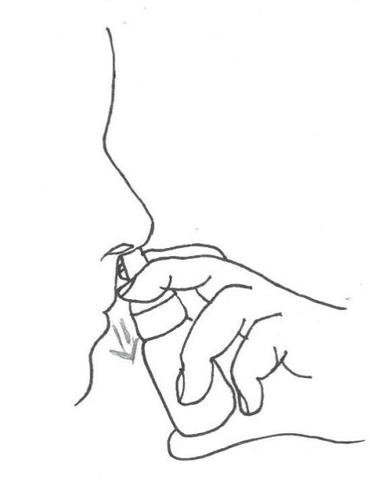
When using Azelamed, the patient should keep their head in an upright position (see instructions (diagrams) below).
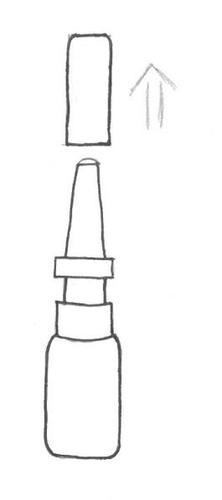
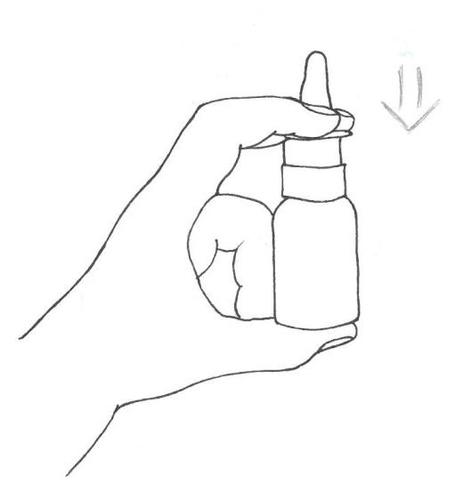
- 1. Remove
- 2. … before first use
- 3. Press the pump a few times (three times) until a uniform spray appears. Then spray once into each nostril, keeping the head straight.
- 4. After use, wipe the tip with a clean tissue and replace the protective cap. Note: If the nasal spray has not been used for three weeks or more and the patient starts using it again, the pump should be pressed a few times before administration.
Duration of treatment
The duration of treatment with Azelamed depends on the type, severity, and progression of symptoms.
Azelamed is suitable for long-term treatment.
If the patient feels that the effect of Azelamed is too strong or too weak, they should consult their doctor or pharmacist.
Using a higher dose of Azelamed than recommended
Azelamed is used locally in the nose. Since one spray contains a small dose of the active substance, the occurrence of overdose symptoms is unlikely, even if a much higher dose than recommended is used locally.
However, if a large amount of this medicine is accidentally ingested (e.g., if a child swallows the entire contents of the bottle), the patient should contact their doctor.
There is no experience with the use of toxic (very high, poisonous) doses of azelastine hydrochloride in humans. Based on animal studies, after significant overdose or in case of poisoning, symptoms from the central nervous system (e.g., restlessness, excitement, or deep, prolonged drowsiness or sleepiness) can be expected. In such cases, symptomatic treatment should be used.
Missing a dose of Azelamed
If a dose of Azelamed is missed, no specific action should be taken.
Treatment should be continued with the same dose when the next dose is due. If necessary, Azelamed can also be used between the missed dose and the next scheduled dose.
Stopping treatment with Azelamed
If possible, Azelamed should be used regularly until the symptoms disappear.
If treatment with Azelamed is stopped, there is a risk of recurrence of typical disease symptoms.
In case of doubts about the use of this medicine, the patient should consult their doctor or pharmacist.
4. Possible side effects
Like all medicines, Azelamed can cause side effects, although not everybody gets them.
Frequent (may affect up to 1 in 10 people):
Bitter taste in the mouth, usually caused by incorrect position during administration (head tilted back during administration, see section 3 "Method of administration"), which may sometimes cause nausea.
Uncommon (may affect up to 1 in 100 people):
Irritation of the inflamed nasal mucosa (burning, tingling), sneezing, nosebleeds.
Rare (may affect up to 1 in 1,000 people):
Nausea.
Very rare (may affect up to 1 in 10,000 people):
Hypersensitivity reactions, drowsiness (dulling, lethargy), skin rash, itching, urticaria, fatigue (exhaustion, tiredness), dizziness, or weakness, which may also be caused by the disease.
What to do
The above side effects are usually temporary. Therefore, no specific action is necessary.
If a bitter taste in the mouth occurs after using Azelamed, it can be counteracted by drinking a non-alcoholic beverage (e.g., juice, milk).
Reporting side effects
If any side effects occur, including any possible side effects not listed in the leaflet, the patient should inform their doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, more information can be collected on the safety of the medicine.
5. How to store Azelamed
The medicine should be stored out of sight and reach of children.
This medicine should not be used after the expiry date stated on the label and carton after "EXP". The expiry date refers to the last day of the month stated.
Store in an upright position.
Shelf life after first opening the package
After first opening the bottle, the product Azelamed should not be used for more than 6 months.
Medicines should not be disposed of via wastewater. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Azelamed contains:
- The active substance is azelastine hydrochloride. 1 ml of solution contains 1 mg of azelastine hydrochloride. Each spray (0.14 ml) contains 0.14 mg of azelastine hydrochloride.
- The other ingredients are: disodium edetate, anhydrous citric acid, disodium phosphate dodecahydrate, sodium chloride, hypromellose 4000, purified water.
What Azelamed looks like and contents of the pack
Azelamed is a clear, colorless to slightly yellowish nasal spray solution (nasal administration).
Azelamed is available in a brown glass bottle with a pump spray containing an applicator and protective cap.
Pack size:
10 ml solution in a 10 ml bottle.
Marketing authorization holder and manufacturer:
Marketing authorization holder
SUN-FARM Sp. z o.o.
ul. Dolna 21
05-092 Łomianki
Manufacturer
mibe GmbH Arzneimittel
Münchener Straße 15
06796 Brehna
Germany
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Countries:
Austria:
Azedil 1 mg/ml Nasenspray
Croatia:
Azelamed 1 mg/ml sprej za nos, otopina
Germany:
Azedil 1 mg/ml Nasenspray, Lösung
Poland:
Azelamed
Date of last revision of the leaflet:09.2022
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- Importermibe GmbH Arzneimittel
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AzelamedDosage form: Aerosol, 1 mg/mlActive substance: azelastineManufacturer: Galenicum Health, S.L.U. SAG Manufacturing S.L.U.Prescription not requiredDosage form: Aerosol, 1.5 mg/mlActive substance: azelastinePrescription not requiredDosage form: Aerosol, 1 mg/ml (0.1%)Active substance: azelastinePrescription not required
Alternatives to Azelamed in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Azelamed in Spain
Alternative to Azelamed in Ukraine
Online doctors for Azelamed
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Azelamed – subject to medical assessment and local rules.








