AFLUON 1 mg/ml NASAL SPRAY SOLUTION

How to use AFLUON 1 mg/ml NASAL SPRAY SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
PACKAGE LEAFLET: INFORMATION FOR THE USER
AFLUON 1 mg/ml nasal spray solution
Azelastine hydrochloride
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others, even if they have the same symptoms as you, as it may harm them.
- If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet.
Contents of the pack:
- What Afluon is and what it is used for
- What you need to know before you use Afluon
- How to use Afluon
- Possible side effects
5 Conservation of Afluon
- Contents of the pack and further information
1. What AFLUON is and what it is used for
Afluon contains azelastine, which belongs to a group of medicines that prevent the effects of histamine (antihistamines) and other substances that the body produces as part of an allergic reaction, which cause symptoms of sneezing, runny nose, itching or nasal congestion. Azelastine also has an additional anti-inflammatory effect.
Afluon is used for the treatment of symptoms of seasonal allergic rhinitis and acute exacerbations (attacks) of perennial allergic rhinitis.
2. What you need to know before you use AFLUON
Do not use Afluon
-if you are allergic to azelastine or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor or pharmacist before starting to use Afluon
Use of AFLUON with other medicines.
Tell your doctor or pharmacist that you are using, have recently used, or might use any other medicines.
No specific interactions have been studied.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Due to the nasal route of administration and the low doses administered, minimal systemic exposure can be expected. However, as with all medicines, precautions should be taken during use in pregnancy and breastfeeding.
Driving and using machines
No effects on the ability to drive or use machines have been described with the use of Afluon.
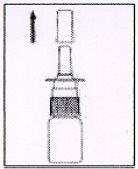
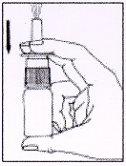
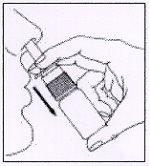
3. How to use AFLUON
Follow exactly the administration instructions of this medicine indicated by your doctor or pharmacist. In case of doubt, consult your doctor or pharmacist again.
The recommended dose in adults is one spray (0.14 ml) in each nostril, twice a day (0.56 mg of azelastine hydrochloride).
Use in children over 6 years old: one spray (0.14 ml) in each nostril, twice a day (0.56 mg of azelastine hydrochloride).
Use in elderly people: No specific studies have been conducted.
Instructions for the correct administration of the medicine
|
|
|
|
|
|
- Clean and replace the protective cap.
If you use more AFLUON than you should
If you have administered too much Afluon, consult your doctor or pharmacist.
With the nasal route of administration, no overdose reactions are expected.
Animal studies show that toxic doses can produce symptoms on the Central Nervous System (excitement, tremors, convulsions). If this occurs in humans, symptomatic and supportive treatment will be initiated. If the overdose is recent, gastric lavage is recommended.
In case of overdose or accidental ingestion, consult the Toxicology Information Service. Telephone: (91) 562 04 20.
If you forget to use AFLUON
Do not use a double dose to make up for forgotten doses.
If you forget to use your medicine, use it as soon as you remember and put the next dose 12 hours later, if necessary.
If you stop treatment with Afluon
Do not stop treatment abruptly.
If you have any other questions about the use of this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Afluon can cause side effects, although not everybody gets them.
Common side effects (affect 1 to 10 people out of 100): After administration, a bitter taste may appear due to incorrect application, for example, with the head too far back. Occasionally, this bitter taste can cause nausea.
Uncommon side effects (affect 1 to 10 people out of 1000): irritation of the nasal mucosa, such as burning, itching, sneezing.
In isolated cases: epistaxis (small nasal hemorrhages).
If you experience any side effects, talk to your doctor or pharmacist, even if they are not listed in this leaflet.
5. Conservation of AFLUON
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiry date is the last day of the month shown.
Do not store below 8°C. Do not refrigerate.
Medicines should not be disposed of via wastewater or household waste. Dispose of the packaging and any unused medicine in the pharmacy's SIGRE point. If you are unsure, ask your pharmacist how to dispose of the packaging and any unused medicine. This will help protect the environment.
6. Contents of the pack and further information
Composition ofAfluon
- The active substance is azelastine hydrochloride 1 mg per ml.
- The other ingredients are: disodium edetate, hypromellose, citric acid, sodium phosphate, sodium chloride, and purified water.
Appearance and pack contents
Afluon is a colorless and transparent solution.
Afluon is available in 10 ml and 20 ml bottles, provided with a dosing valve, which contains a solution for nasal spray.
Marketing authorisation holder
Cooper Consumer Health B.V.
Verrijn Stuartweg 60,
1112 AX Diemen,
Netherlands
Manufacturer
Madaus GmbH
Lütticher Str. 5
53842 Troisdorf
Germany
You can request more information about this medicine by contacting the local representative of the marketing authorisation holder:
Vemedia Pharma Hispania, S.A.
C/ Aragón, 182, 5th floor
08011 - Barcelona
Spain
Date of last revision of this leaflet: February 2021
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Average pharmacy price7.79 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AFLUON 1 mg/ml NASAL SPRAY SOLUTIONDosage form: NASAL PRODUCT, 1.5 mg/mlActive substance: azelastineManufacturer: Cooper Consumer Health B.V.Prescription requiredDosage form: NASAL PRODUCT, 1.5 mg/mlActive substance: azelastineManufacturer: Cooper Consumer Health B.V.Prescription requiredDosage form: NASAL PRODUCT, 1 mg/mlActive substance: azelastineManufacturer: Zentiva K.S.Prescription required
Online doctors for AFLUON 1 mg/ml NASAL SPRAY SOLUTION
Discuss questions about AFLUON 1 mg/ml NASAL SPRAY SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions