

Astepro

Ask a doctor about a prescription for Astepro

How to use Astepro
Package Leaflet: Information for the Patient
Astepro, 1.5 mg/mL, Nasal Spray, Solution
Azelastine Hydrochloride
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
This medicine should always be used exactly as described in this package leaflet or as directed by your doctor or pharmacist.
- Keep this leaflet, you may need to read it again.
- If you need advice or further information, ask your pharmacist.
- If you experience any side effects, including any not listed in this leaflet, tell your doctor or pharmacist. See section 4.
- If after 2 to 3 days of treatment, there is no improvement or you feel worse, you should contact your doctor.
Table of Contents of the Package Leaflet
- 1. What is Astepro and what is it used for
- 2. Important information before using Astepro
- 3. How to use Astepro
- 4. Possible side effects
- 5. How to store Astepro
- 6. Contents of the pack and other information
1. What is Astepro and what is it used for
Astepro, nasal spray contains azelastine hydrochloride, a substance belonging to the group of antihistamine drugs. Antihistamine drugs prevent the action of histamine produced by the body as a result of an allergic reaction.
Astepro, nasal spray is used to treat symptoms of allergic rhinitis in adults, adolescents, and children aged 6 and older. Allergic rhinitis is a type of allergic reaction to allergens such as plant pollen, dust mites, or animal dander.
Astepro, nasal spray reduces the severity of symptoms such as runny nose, sneezing, itching, or stuffy nose.
If after 2 to 3 days of treatment, there is no improvement or you feel worse, you should contact your doctor. Do not use the medicine for more than 4 weeks without consulting your doctor.
2. Important information before using Astepro
When not to use Astepro
- if you are allergic to azelastine hydrochloride or any of the other ingredients of this medicine (listed in section 6),
- in children under 6 years of age.
Warnings and precautions
Before starting treatment with Astepro, you should discuss it with your doctor or pharmacist.
Children
Astepro should not be used in children under 6 years of age.
Astepro and other medicines
Tell your doctor about all the medicines you are taking now or have taken recently, and about the medicines you plan to take.
No studies have been conducted on the interaction of Astepro nasal spray with other medicines.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, ask your doctor or pharmacist for advice before using this medicine.
There are limited data on the use of Astepro during pregnancy and breastfeeding.
Driving and using machines
Astepro has a minor influence on the ability to drive and use machines.
Rarely, there may be a feeling of fatigue, tiredness, exhaustion, dizziness, or weakness, which may be caused by the disease itself or the use of Astepro. In such cases, do not drive or operate machinery. Be aware that drinking alcohol may increase this effect.
3. How to use Astepro
This medicine should always be used exactly as described in this package leaflet or as directed by your doctor or pharmacist. If you are unsure, ask your doctor or pharmacist.
Astepro is intended for use in the nose only.
Important:
If you have any doubts about using the medicine, ask your doctor or pharmacist. If you are unsure about the dose to use or how to use the medicine, ask your doctor or pharmacist.
The duration of treatment depends on the type, duration, and course of symptoms and is determined by your doctor. Astepro nasal spray may only be used in people with allergic rhinitis diagnosed by a doctor and according to the principles determined by the doctor.
Using the medicine for more than 4 weeks should be done under medical supervision.
Adults and adolescents (12 years and older):
It is recommended to use two sprays in each nostril once a day.
In some cases, it may be necessary to administer two sprays in each nostril twice a day. The maximum daily dose is two sprays in each nostril twice a day.
Children aged 6 to 11:
It is recommended to use one spray in each nostril twice a day.
Astepro nasal spray is not recommended for use in children under 6 years of age due to the lack of data on safety and/or efficacy in this age group.
If possible, Astepro should be used regularly until the symptoms disappear. If treatment is interrupted, the symptoms of the disease may worsen.
Astepro nasal spray is intended for long-term use, but without consulting a doctor, the medicine should not be used for more than 4 weeks. If after 2 to 3 days of treatment, there is no improvement or you feel worse, you should contact your doctor.
Using the medicine for more than 4 weeks is not recommended in children aged 6 to 11 due to the lack of clinical data.
Method of administration
- 1. Clean the nose.
- 2. Remove the protective cap (Figure 1).
- 3. Before the first use, prime the pump by pressing and releasing the pump six times (6 times) releasing a dose of medicine into the air (Figure 2). If Astepro nasal spray has not been used for 3 or more days, re-prime the pump by pressing and releasing until a fine spray appears.
- 4. While pressing the pump, spray a single dose of medicine into each nostril, keeping your head in an upright position. Do not tilt your head back (Figure 3).
- 5. Wipe the tip of the pump with a tissue and replace the protective cap.
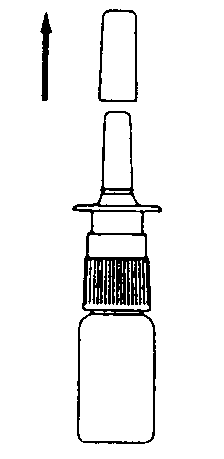
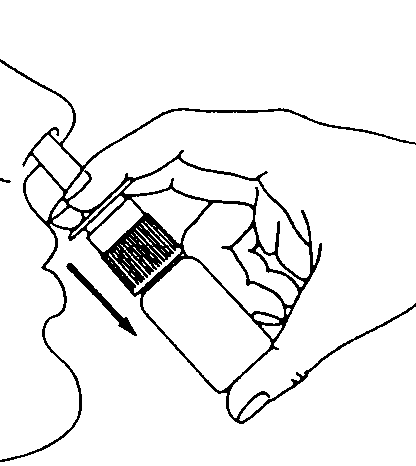
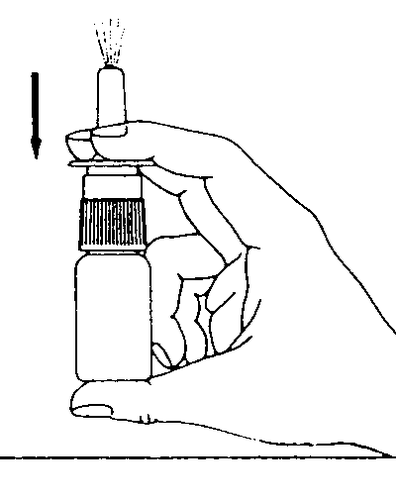
Figure 1 Figure 2 Figure 3
Using a higher dose of Astepro than recommended
In the event of using too much medicine, there is a small likelihood of complications. After an overdose or accidental ingestion, you can expect central nervous system disorders (including drowsiness, disorientation, coma, rapid heartbeat, low blood pressure). If you are unsure, ask your doctor. If someone, especially a child, accidentally ingests Astepro, you should immediately contact a doctor or the nearest hospital emergency department.
Missing a dose of Astepro
You should use the nasal spray as soon as you remember, and then continue with the next dose at the usual time. Do not use a double dose to make up for the missed dose.
If you have any further doubts about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Astepro can cause side effects, although not everybody gets them.
After using this medicine, a very rare allergic reaction may occur, which manifests as swelling of the face, tongue, or throat, swallowing problems, hives, wheezing, or difficulty breathing. In such a case, you should stop using the medicine immediately and consult a doctor or go to the hospital.
Common(may affect up to 1 in 10 people):
- bitter taste in the mouth, especially if you tilt your head back while spraying the nasal spray. If you experience a bitter taste in your mouth after using Astepro nasal spray, you can neutralize it by drinking a non-alcoholic beverage (such as juice, milk).
Uncommon(may affect up to 1 in 100 people):
- mild irritation inside the nose. This may cause a burning sensation, itching, bleeding from the nose, and sneezing.
Rare(may affect up to 1 in 1,000 people):
- unpleasant taste, which may cause nausea. You may experience a feeling of fatigue (tiredness, exhaustion), dizziness, weakness, or drowsiness, which may also be caused by the disease itself.
Very rare(may affect up to 1 in 10,000 people):
- allergic reactions (hypersensitivity), rash, itching, or hives.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, tell your doctor or pharmacist. Side effects can be reported directly to the Department of Drug Safety Monitoring of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Aleje Jerozolimskie 181C, 02-222 Warsaw, tel.: 22 49-21-301, fax: 22 49-21-309,
website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Astepro
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the label and carton. The expiry date refers to the last day of that month.
Shelf-life after first opening the bottle: 6 months. After this period, any unused medicine should be discarded.
Do not store in the refrigerator or freeze.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Astepro contains
- The active substance is azelastine hydrochloride. 1 mL of solution contains 1.5 mg of azelastine hydrochloride. 1 dose, i.e., one actuation of the pump (0.14 mL) delivers 0.21 mg of azelastine hydrochloride, equivalent to 0.19 mg of azelastine.
- The other ingredients are: hypromellose, sucralose, liquid sorbitol, crystallizing, disodium edetate, sodium citrate, purified water.
What Astepro looks like and contents of the pack
Astepro, nasal spray, solution is a clear, colorless solution.
Astepro, nasal spray, solution is available in a brown glass bottle with a pump dispenser made of PP, PE, POM, elastomer, and stainless steel with a protective cap, in a cardboard box.
Bottle containing 5 mL of solution.
Bottle containing 10 mL of solution.
Bottle containing 17 mL of solution.
Bottle containing 20 mL of solution.
Bottle containing 22 mL of solution.
Not all pack sizes may be marketed.
Marketing authorization holder:
Cooper Consumer Health B.V.
Verrijn Stuartweg 60
1112AX Diemen
Netherlands
Manufacturer:
Madaus GmbH
Lütticher Straße 5
53842 Troisdorf
Germany
For more information, please contact:
Viatris Healthcare Sp. z o.o.
tel. 22 546 64 00
Date of last revision of the package leaflet:
- Country of registration
- Active substance
- Prescription requiredNo
- ImporterMADAUS GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to AsteproDosage form: Aerosol, 1 mg/mlActive substance: azelastineManufacturer: Galenicum Health, S.L.U. SAG Manufacturing S.L.U.Prescription not requiredDosage form: Aerosol, 1.5 mg/mlActive substance: azelastinePrescription not requiredDosage form: Aerosol, 1 mg/ml (0.1%)Active substance: azelastinePrescription not required
Alternatives to Astepro in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Astepro in Spain
Alternative to Astepro in Ukraine
Online doctors for Astepro
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Astepro – subject to medical assessment and local rules.








