
ASTEPRO 1.5 mg/ml NASAL SPRAY SOLUTION

How to use ASTEPRO 1.5 mg/ml NASAL SPRAY SOLUTION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for theuser
Astepro 1.5 mg/ml nasal spray solution
azelastine hydrochloride
Read all of this leaflet carefully before you start usingthis medicine, because it contains important information for you.
- Keep this leaflet, you may need to read it again
- If you have any further questions, ask your doctor or pharmacist
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours
- If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack
- What Astepro is and what it is used for
- What you need to know before you use Astepro
- How to use Astepro
- Possible side effects
- Storage of Astepro
- Contents of the pack and further information
1. What Astepro is and what it is used for
Astepro contains azelastine hydrochloride, which belongs to a group of medicines called antihistamines. Antihistamines work by preventing the effects of histamine, which is produced by the body as part of an allergic reaction.
Astepro is used for the treatment of seasonal allergic rhinitis (hay fever) in adults, adolescents, and children aged 6 years and older. Allergic rhinitis is an allergic reaction to substances such as pollen.
It usually causes runny nose, sneezing, itching, or nasal congestion. Astepro can help you control these symptoms.
2. What you need to know before you use Astepro
Do not use Astepro
- If you are allergic to azelastine hydrochloride or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist before you start using Astepro.
Children
Astepro is not recommended for children under 6 years of age.
Using Astepro with other medicines
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Pregnancy, breastfeeding, and fertility
There is only limited information about the effects of Astepro on the fetus or baby.
If you are pregnant or breastfeeding, think you might be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
Astepro has a negligible influence on the ability to drive and use machines.
You may rarely experience fatigue or dizziness due to the disease itself or when using Astepro. In these cases, do not drive or use machines. Consuming alcohol may intensify these effects.
3. How to use Astepro
Follow exactly the administration instructions of this medicine given by your doctor. If you are unsure, consult your doctor or pharmacist again.
Adults and adolescents over 12 years
- The recommended dose is two sprays in each nostril once a day. In some cases, two sprays in each nostril twice a day may be required.
- The maximum daily dose is two sprays in each nostril twice a day.
Use in children from 6 to 11 years
The recommended dose is one spray in each nostril twice a day.
Clinical experience over 4 weeks showed good efficacy and safety in children. There is no experience in children for longer than this; however, clinical trials of up to one year's duration using twice the daily dose showed good safety in adults and adolescents.
This medicine is not recommended for use in children under 6 years of age due to the lack of safety and/or efficacy data.
Duration of treatmentIf possible, use this medicine regularly until your symptoms have disappeared. If you stop using Astepro, it is likely that your symptoms will return.
This medicine is suitable for long-term use.
It is not recommended to use it for more than 4 weeks in children from 6-11 years due to the lack of clinical data.
Method of administration
For nasal use.
How to use the spray
- Blow your nose first.
- Remove the protective cap (Figure 1).
- Before using for the first time, the pump must be pressed six times until a constant spray is produced (Figure 2). When Astepro has not been used for three or more days, the pump must be pressed a sufficient number of times until a fine spray appears.
- Spray once in each nostril, keeping your head upright. Do not tilt your head back (Figure 3).
- Clean and replace the protective cap.

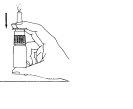
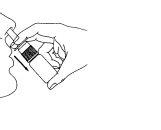
Figure 1 Figure 2 Figure 3
If you use more Astepro than you should
If you apply too much Astepro to your nose, you are unlikely to have any problems. If you are concerned, talk to your doctor.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately or call the Toxicology Information Service, phone 91 562 04 20, stating the medicine and the amount ingested.
If you forget to use Astepro
If you forget to use your medicine, use it as soon as you remember, then apply the next dose at your usual time. Do not use a double dose to make up for forgotten doses.
If you have any questions about using this medicine, talk to your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
These effects include:
Common (may affect up to 1 in 10 people):
- Unpleasant taste in your mouth (especially if you tilt your head too far back during administration of the nasal spray).
Uncommon (may affect up to 1 in 100 people):
- Mild irritation inside the nose (itching, pruritus), sneezing, and blood in the nose.
Rare (may affect up to 1 in 1,000 people):
- The unpleasant taste may make you feel unwell. Fatigue (tiredness, exhaustion), dizziness, weakness, or a feeling of drowsiness that may appear may also be caused by the disease itself.
Very rare (may affect up to 1 in 10,000 people):
- An allergic reaction, skin rash, itching, or hives.
Reporting of side effects:
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. You can also report side effects directly through the Spanish Medicines Agency's website: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Astepro
Keep out of the sight and reach of children.
Do not use your spray after the expiry date stated on the carton and label after "EXP". The expiry date is the last day of the month stated.
Discard any remaining medicine 6 months after opening the nasal spray.
Do not refrigerate or freeze.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and further information
Composition of Astepro
- The active substance is azelastine hydrochloride. 1 ml of solution contains 1.5 mg of azelastine hydrochloride. Each spray (0.14 ml) contains 0.21 mg of azelastine hydrochloride, equivalent to 0.19 mg of azelastine.
- The other ingredients are: hypromellose, sucralose, sorbitol liquid (crystallizable), disodium edetate, sodium citrate, purified water.
Appearance and pack contents
Astepro is a clear, colorless solution.
It is available in a brown glass bottle with a metering pump. The 10 ml bottle contains 5 or 10 ml of nasal spray solution. The 20 ml bottle contains 17, 20, or 22 ml of nasal spray solution.
Not all pack sizes may be marketed.
Marketing authorisation holder and manufacturer
Marketing authorisation holder
Cooper Consumer Health B.V.
Verrijn Stuartweg 60,
1112 AX Diemen,
Netherlands
Manufacturer
Madaus GmbH
Lütticher Straße 5
53842 Troisdorf
Germany
You can obtain further information on this medicine by contacting the local representative of the marketing authorisation holder:
Vemedia Pharma Hispania, S.A.
C/ Aragón, 182, 5ª planta
08011 - Barcelona
Spain
This medicine is authorised in the Member States of the EEA under the following names:
Austria: Astepro 1.5 mg/ml Nasenspray
Denmark: Astepro
Finland: Astepro 0.19 mg/annos nenäsumute, liuos
Germany: Astepro 1,5 mg/ml Nasenspray, Lösung
Ireland: Astepro 1.5 mg/ml Nasal Spray, Solution
Spain: Astepro 1,5 mg/ml solución para pulverización nasal
Italy: Afluon
Netherlands: Astepro 0.15 %
Sweden: Astepro 0.19 mg/dos nässpray, lösning
United Kingdom: Astepro 0.15 % Nasal Spray
Date of last revision of thisleaflet:January 2022
Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ASTEPRO 1.5 mg/ml NASAL SPRAY SOLUTIONDosage form: NASAL PRODUCT, 0.1 g azelastine hydrochloride/100 mlActive substance: azelastineManufacturer: Cooper Consumer Health B.V.Prescription requiredDosage form: NASAL PRODUCT, 1.5 mg/mlActive substance: azelastineManufacturer: Cooper Consumer Health B.V.Prescription requiredDosage form: NASAL PRODUCT, 1 mg/mlActive substance: azelastineManufacturer: Zentiva K.S.Prescription required
Online doctors for ASTEPRO 1.5 mg/ml NASAL SPRAY SOLUTION
Discuss questions about ASTEPRO 1.5 mg/ml NASAL SPRAY SOLUTION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions











