
ATROALDO 20 micrograms/PUFF pressurized inhalation solution

How to use ATROALDO 20 micrograms/PUFF pressurized inhalation solution
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the User
ATROALDO 20 micrograms/puff inhalation solution in a pressurized container
ipratropium bromide
Read this package leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this package leaflet, you may need to read it again. If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others, as it may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor or pharmacist, even if they are not mentioned in this package leaflet. See section 4.
Contents of the package leaflet
- What is ATROALDO and what is it used for
- What you need to know before you start using ATROALDO
- How to use ATROALDO
- Possible side effects
- Storage of ATROALDO
- Contents of the pack and further information
1. What is ATROALDO and what is it used for
ATROALDO contains ipratropium bromide as the active substance and belongs to a group of medicines called inhalation bronchodilators.
It is used for the maintenance treatment of bronchospasm associated with chronic obstructive pulmonary diseases (COPD), including chronic bronchitis and emphysema.
2. What you need to know before you start using ATROALDO
Do not useATROALDOif:
- If you are allergic to atropine, its derivatives (e.g. tiotropium), or any of the other components of this medicine (listed in section 6).
- If you experience acute attacks of coughing, wheezing, and difficulty breathing (bronchospasm) that require a rapid response.
Warnings and precautions
Talk to your doctor or pharmacist before you start using this medicine:
- If you have cystic fibrosis, as you may be more prone to gastrointestinal motility disorders.
- If you experience acute difficulty breathing that worsens rapidly. You should consult your doctor immediately.
- Immediate allergic reactions, such as hives, angioedema, skin rash, cough, wheezing, and difficulty breathing (bronchospasm), swelling of the mouth and throat (oropharyngeal edema), and generalized allergic reaction (anaphylaxis) may occur.
- If you have prostatic hyperplasia or obstruction of the neck of the bladder.
- If you are prone to increased eye pressure (narrow-angle glaucoma).
- If you spray the solution into your eyes, eye complications such as pupil dilation, increased intraocular pressure, narrow-angle glaucoma, eye pain may occur, so it is essential to follow your doctor's instructions strictly for administration.
- If you experience a combination of eye symptoms such as pain or discomfort, blurred vision, halos, or colored images, along with redness of the eyes, these may be signs of narrow-angle glaucoma, so you should consult a doctor.
Other medicines and ATROALDO
Tell your doctor or pharmacist if you are using or have recently used other medicines, including those obtained without a prescription.
Beta-adrenergics and xanthine derivatives may enhance the bronchodilator effect. ATROALDO may increase the anticholinergic effects of other medicines.
This medicine can be administered together with other commonly used medicines for the treatment of chronic obstructive pulmonary disease, including sympathomimetic bronchodilators, methylxanthines, steroids, and disodium cromoglycate, without the appearance of harmful interactions.
Pregnancy and breastfeeding:
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
The safety of this medicine during pregnancy has not been established. The benefit of using it should be carefully weighed against the potential risk to the fetus, so the usual precautions should be taken when using medicines during this period.
It is not known whether this medicine is excreted in breast milk. However, it is unlikely that this medicine can be ingested by the infant in significant amounts, especially since the preparation is administered by inhalation. Nevertheless, since many medicines are excreted in breast milk, it should be administered with caution to breastfeeding women.
Driving and using machines:
No harmful effects on driving have been reported.
ATROALDO contains ethanol
This medicine contains about 8.4 mg of alcohol (ethanol) per inhalation. The amount per inhalation of this medicine is equivalent to less than 1 ml of beer or 1 ml of wine. The small amount of alcohol in this medicine does not produce any noticeable effect.
3. How to use ATROALDO
Instructions for proper use:
Follow your doctor's or pharmacist's instructions for administering this medicine exactly. If you are in doubt, consult your doctor or pharmacist again.
The dosage should be adjusted individually.
For adults and children over 6 years, the following dosage is recommended:
2 inhalations (equivalent to 40 micrograms of anhydrous ipratropium bromide), 4 times a day.
Since the need for higher doses suggests that additional treatment with another medicine may be necessary, a daily total dose of more than 12 inhalations (240 micrograms of anhydrous ipratropium bromide) should not be exceeded.
You should consult your doctor whenever you do not achieve significant improvement or your condition worsens, in order to determine a new therapeutic program. Also, you should consult your doctor promptly in case of significant difficulty breathing or when difficulty breathing worsens rapidly.
In children, this medicine should only be administered under medical supervision and always under adult supervision.
Your doctor will indicate the duration of your treatment with this medicine. Do not stop treatment before, as your doctor is the person to give you precise instructions.
Instructions for the correct administration of the preparation:
This medicine is administered by inhalation. The correct handling of the inhalation solution is crucial for the success of the treatment.
Before each application, the following guidelines will be observed:
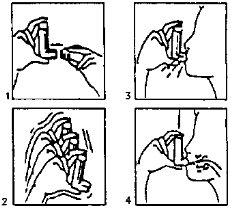
- Remove the protective cap (fig. 1). If it is a new inhaler or has not been used for several days, shake the container (fig. 2) and perform a puff to ensure the inhaler is working properly. If the inhaler is used regularly, proceed to the next instructions:
B Shake the inhaler.
C Eliminate as much air as possible from your lungs.
- Attach the inhaler to your mouth according to the position indicated in the drawing (fig. 3).
- Make a deep inhalation.
You should press, according to the arrows in the drawing (fig. 4), the device while making this inhalation.
- Remove the inhaler from your mouth and try to hold your breath for a few seconds and then exhale slowly.
- You should periodically wash the plastic mouthpiece. To do this, remove the actuator from the inhaler and rinse it with plenty of water.
- Store with the protective cap in place to protect it from dust and dirt.
- It is recommended to rinse your mouth with water after each inhalation.
If you think the effect of this medicine is too strong or too weak, tell your doctor or pharmacist.
If you use more ATROALDO than you should
Symptoms such as dry mouth, visual accommodation disorders, and tachycardia may occur.
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately, or call the Toxicology Information Service, phone 915620420, indicating the medicine and the amount ingested.
If you forget to take ATROALDO
Do not take a double dose to make up for forgotten doses.
If you have any further questions about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The following side effects have been reported:
Common(affect 1 in 10 people)
- Headache
- Dizziness
- Cough
- Pharyngitis
- Paradoxical bronchospasm (obstruction of the airways)
- Dry mouth
- Gastrointestinal motility disorders (e.g. constipation, diarrhea, and vomiting).
Uncommon(affect less than 1 in 100 people)
- Urticaria (including giant urticaria), rash, and pruritus
- Visual accommodation disorders, narrow-angle glaucoma (increased eye pressure)
- Increased heart rate
Rare(affect less than 1 in 1000 people)
- Anaphylactic reactions, swelling of the tongue, lips, and face (angioedema)
- Increased intraocular pressure, eye pain, pupil dilation (mydriasis)
- Palpitations, supraventricular tachycardia, atrial fibrillation
- Laryngeal spasm
- Nausea
- Urinary retention
If you think any of the side effects you are experiencing are serious, or if you notice any side effects not mentioned in this package leaflet, tell your doctor or pharmacist.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist, even if they are not mentioned in this package leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of ATROALDO
Keep this medicine out of the sight and reach of children.
Store below 30°C. Store in the original packaging to protect it from light. Do not freeze.
If the inhaler is very cold, remove the cartridge and warm it with your hand for a few minutes before use. Do not use any other method to heat it.
The container is under pressure. It should not be pierced, broken, or burned, even if it appears to be empty.
Do not use ATROALDO after the expiry date stated on the container after EXP. The expiry date is the last day of the month indicated.
Medicines should not be disposed of via wastewater or household waste. Place the containers and medicines you no longer need in the pharmacy's SIGRE collection point. If you have any doubts, ask your pharmacist how to dispose of the containers and medicines you no longer need. This will help protect the environment.
6. Contents of the pack and further information
Composition ofATROALDO:
- The active substance is ipratropium bromide.
- The other components (excipients) are anhydrous citric acid, purified water, ethanol, and Norflurane.
Each inhalation contains 21 micrograms of ipratropium bromide monohydrate (equivalent to 20 micrograms of anhydrous ipratropium bromide).
Appearance of the product and contents of the pack
ATROALDO is a clear, colorless solution presented in a 10 ml container (200 doses) with a dosing valve and oral adapter.
The clinical container is a box containing 20 containers of 10 ml with 200 doses per container.
Marketing authorization holder and manufacturer
Laboratorio Aldo-Unión, S.L.
Baronesa de Maldá, 73
08950 Esplugues de Llobregat (Barcelona)
Spain
Date of the last revision of this package leaflet:July 2010.
Detailed and updated information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/
- Country of registration
- Average pharmacy price4.46 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to ATROALDO 20 micrograms/PUFF pressurized inhalation solutionDosage form: PULMONARY INHALATION, 20 µgActive substance: ipratropium bromideManufacturer: Boehringer Ingelheim Espana S.A.Prescription requiredDosage form: PULMONARY INHALATION, 250 µgActive substance: ipratropium bromideManufacturer: Boehringer Ingelheim Espana S.A.Prescription requiredDosage form: PULMONARY INHALATION, 500 µgActive substance: ipratropium bromideManufacturer: Boehringer Ingelheim Espana S.A.Prescription required
Online doctors for ATROALDO 20 micrograms/PUFF pressurized inhalation solution
Discuss questions about ATROALDO 20 micrograms/PUFF pressurized inhalation solution, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















