
Ipravent Inhaler

Ask a doctor about a prescription for Ipravent Inhaler

How to use Ipravent Inhaler
Leaflet accompanying the packaging: patient information
Ipravent Inhaler, 20 micrograms/dose, inhalation aerosol, solution
Ipratropium bromide
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor, pharmacist, or nurse.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. See section 4.
Table of contents of the leaflet
- 1. What Ipravent Inhaler is and what it is used for
- 2. Important information before using Ipravent Inhaler
- 3. How to use Ipravent Inhaler
- 4. Possible side effects
- 5. How to store Ipravent Inhaler
- 6. Contents of the packaging and other information
1. What Ipravent Inhaler is and what it is used for
Ipravent Inhaler contains ipratropium bromide. It belongs to a group of medicines called bronchodilators.
It is used to make breathing easier for adults with chronic asthma or chronic obstructive pulmonary disease (COPD).
Ipravent Inhaler works by opening up the airways.
2. Important information before using Ipravent Inhaler
When not to use Ipravent Inhaler:
The medicine should not be used if the patient has any of the above conditions. In case of any doubts before using Ipravent Inhaler, consult a doctor or pharmacist.
Warnings and precautions
Before starting to use Ipravent Inhaler, discuss with a doctor or pharmacist:
In case of doubts whether the above conditions apply to the patient, before using Ipravent Inhaler, consult a doctor or pharmacist.
Ipravent Inhaler may rarely cause allergic reactions immediately after use. They can take the form of hives or swelling of the upper airways.
In case of any of the following symptoms (angioedema), stop using Ipravent Inhaler and consult a doctor:
- swelling of the face, tongue, or throat,
- difficulty swallowing,
- hives and difficulty breathing.
If breathing difficulties do not improve or worsen despite treatment, always consult a doctor. In case of sudden worsening of lung function, consult a doctor immediately.
Ipravent Inhaler may cause a feeling of dryness in the mouth, which, in the case of long-term treatment, may lead to changes in the teeth and oral mucosa. Brush your teeth with fluoride toothpaste twice a day.
Children and adolescents
This medicine is not intended for use in children and adolescents.
Ipravent Inhaler and other medicines
Tell a doctor or pharmacist about all medicines the patient is currently taking or has recently taken, as well as any medicines the patient plans to take. This includes herbal medicines.
This is because Ipravent Inhaler may affect the action of other medicines. Other medicines may also affect the action of Ipravent Inhaler.
In particular, tell a doctor or pharmacist about the use (current or past) of:
- any other inhaled medicines that make breathing easier, such as salbutamol in an inhaler.
- medicines called "xanthines" that make breathing easier, such as theophylline or aminophylline.
In case of doubts whether the above conditions apply to the patient, before using Ipravent Inhaler, consult a doctor or pharmacist.
Pregnancy, breastfeeding, and fertility
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult a doctor or pharmacist before using this medicine.
As a precaution, it is recommended to avoid using Ipravent Inhaler during pregnancy.
There is no data on the use of this medicine during pregnancy. Before using Ipravent Inhaler during pregnancy, consult a doctor.
It is not known whether Ipravent Inhaler passes into breast milk. Before using Ipravent Inhaler during breastfeeding, consult a doctor.
Driving and using machines
While using Ipravent Inhaler, dizziness, accommodation disorders (difficulty adjusting vision to different distances), vision disorders, or blurred vision may occur. In such a case, do not drive vehicles or use tools and operate machines. The patient should assess whether they can drive vehicles or perform tasks that require attention.
Ipravent Inhaler contains ethanol (alcohol)
This medicine contains 8.4 mg of alcohol (ethanol) in one dose. The amount of alcohol in a dose of this medicine is equivalent to less than 4.3 ml of beer or 1.72 ml of wine.
The small amount of alcohol in this medicine will not have noticeable effects.
3. How to use Ipravent Inhaler
Ipravent Inhaler should always be used as directed by a doctor. In case of doubts, consult a doctor.
To get the best results, follow the instructions below. If you have any doubts after reading these instructions, consult a doctor or pharmacist.
The recommended dose is:
Adults (including the elderly)
- 1-2 inhalations four times a day.
- Maximum 12 inhalations per day.
This medicine should always be used exactly as described in this patient leaflet or as directed by a doctor, pharmacist, or nurse. Do not use more inhalations than recommended by a doctor. In case of doubts, consult a doctor, pharmacist, or nurse.
Checking the inhaler
Before first use, check that the inhaler is working properly. The inhaler should also be checked if it has not been used for 3 days or longer.
- 1. Remove the mouthpiece cover by gently squeezing its sides with your thumb and index finger.
- 2. Point the mouthpiece away from you and press the canister twice (once, then again) to release two doses into the air.
How to use the inhaler
It is essential to breathe as slowly as possible just before using the inhaler.
- 1. Use the inhaler in a standing or sitting position.
- 2. Remove the mouthpiece cover. Check the mouthpiece from the outside and inside to make sure it is clean and free of particles (Figure A).
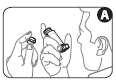
- 3. Hold the inhaler upright, grasping the base of the mouthpiece with your thumb. Exhale slowly until it becomes uncomfortable (Figure B). Do not inhale yet.
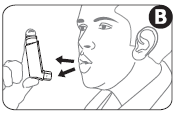
- 4. Place the mouthpiece between your teeth. Close your lips around it. Do not bite (Figure C).
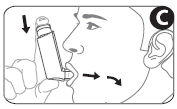
- 5. Inhale slowly through your mouth. As soon as you start inhaling, press the top of the canister to release the dose of medicine. Do this while continuing to breathe slowly and deeply (Figure C).
- 6. Hold your breath, remove the inhaler from your mouth, and remove your finger from the top of the inhaler. Hold your breath for a few seconds or until it becomes uncomfortable (Figure D).
- 7. If your doctor has prescribed two doses, wait about one minute before taking the next dose. Repeat steps 3 to 7.
- 8. After using the inhaler, put the mouthpiece cover back on to prevent dust from entering. Put the cover back on by pushing it firmly.
The mouthpiece is designed specifically for use with this medicine only. Do not use another mouthpiece with this medicine or use the mouthpiece with another medicine.
Practice using the inhaler in front of a mirror several times. If a mist is coming out of the top of the inhaler or the sides of your mouth, try again.
For people with reduced hand strength, it may be more convenient to hold the inhaler with both hands. You should then place both index fingers on the top of the inhaler and both thumbs on the bottom of the inhaler under the mouthpiece. A doctor, nurse, or pharmacist will help the patient.
The inhaler can be used with the Aerochamber Plus spacer. This may be helpful for people who have difficulty synchronizing their breath with the inhaler activation.
In case of difficulty using Ipravent Inhaler, consult a doctor.
When using Ipravent Inhaler, be careful not to get the aerosol into your eyes.
In case the medicine accidentally gets into your eyes, rinse them with running water. If the aerosol accidentally gets into your eyes, you may experience pain, burning, or redness of the eyes, pupil dilation, blurred vision, color vision, or seeing lights. In such a case, consult a doctor for advice. In case of any other eye problems, consult a doctor for advice. The patient may develop glaucoma, which requires immediate treatment.
Cleaning the inhaler
Regular cleaning of the inhaler is essential. Otherwise, it may not work properly.
- Remove the canister and mouthpiece cover.
- Wash and clean the white mouthpiece in warm water with soap.
- Rinse in warm water and let it air dry; do not use heating systems.
- Clean the small hole in the mouthpiece thoroughly.
- After the mouthpiece is dry, put the canister back and replace the cover.
The metal canister must notbe put in water .
Do not take more than the recommended dose of Ipravent Inhaler
Consult a doctor immediately if:
- the patient feels that the inhaler is not working as well as usual;
- the patient needs to use the inhaler more often than prescribed by the doctor.
The doctor may need to check how well the medicine is working. In some cases, the doctor may change the medicine the patient is taking.
Make sure Ipravent Inhaler does not run out suddenly
The inhaler contains 200 doses of medicine. However, it is not possible to determine if the inhaler is empty or if 200 doses have been used. There may still be a small amount of liquid in the canister. Remember to replace the inhaler after 200 doses (usually after 3-4 weeks of regular use) to ensure that each inhalation contains the correct amount of medicine.
Using more than the recommended dose of Ipravent Inhaler
In case of using more than the recommended dose of the medicine, consult a doctor or go to the hospital immediately. Take all inhalers and other medicines the patient is taking (and, if possible, their packaging) with them. In case of taking too much medicine, the patient may experience dryness in the mouth, rapid heartbeat, pupil dilation, difficulty urinating, headache, constipation, and dizziness.
Missing a dose of Ipravent Inhaler
- If the patient forgets to take a dose, they should take it as soon as possible.
- However, if it is almost time for the next dose, skip the missed dose.
- Do not take a double dose to make up for the missed dose.
In case of any further doubts, consult a doctor or pharmacist.
Stopping the use of Ipravent Inhaler
Do not stop using Ipravent Inhaler without consulting a doctor. In case of any further doubts about using this medicine, consult a doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
In case of any of the following serious side effects, consult a doctor immediately – the patient may need urgent medical attention
Do not use the medicine again (unless the doctor advises otherwise):
- If the patient experiences wheezing or other breathing difficulties after using Ipravent Inhaler (may occur less often than in 1 in 100 people).
- Swelling of the mouth and face, sudden difficulty breathing, low blood pressure, or throat constriction. These may be symptoms of an allergic reaction or angioedema (may occur less often than in 1 in 100 people).
- In case of eye problems. The patient may develop glaucoma, which requires immediate treatment (may occur less often than in 1 in 100 people). If the aerosol accidentally gets into the eyes, the patient may experience pain, burning, or redness of the eyes, pupil dilation, blurred vision, color vision, or seeing lights. In such a case, rinse the eyes with running water and consult a doctor.
The following side effects have been reported in people using Ipravent Inhaler.
They are listed as common, uncommon, rare, or unknown.
Common(may occur in less than 1 in 10 people)
- Headache, dizziness.
- Dry mouth, nausea, stomach upset or discomfort, or gastrointestinal disorders.
- Cough and throat irritation after using Ipravent Inhaler.
Uncommon(may occur in less than 1 in 100 people)
- Itching, rash.
- Unexpected chest tightness, throat swelling, dry throat.
- Diarrhea, constipation, or vomiting.
- Herpes simplex infection of the lips and mouth.
- Difficulty urinating, especially if the patient has had such problems before.
Rare(may occur in less than 1 in 1000 people)
- Accommodation disorders (difficulty adjusting vision to different distances)
- Rapid heartbeat
- Atrial fibrillation
- Hives
Unknown frequency (frequency cannot be estimated from the available data)
- Taste changes
- Shortness of breath
- Stuffy nose
- Dryness of the nasal mucosa
Reporting side effects
If the patient experiences any side effects, including any not listed in this leaflet, they should tell their doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products:
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 21 301
Fax: +48 22 49 21 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help gather more information on the safety of this medicine.
5. How to store Ipravent Inhaler
Keep the medicine out of sight and reach of children.
Do not use this medicine after the expiry date stated on the carton and label on the canister after: EXP. The expiry date refers to the last day of the month stated.
Protect from direct sunlight, high temperatures, and frost.
If the inhaler has been exposed to low temperatures, the patient should remove the metal canister from the plastic casing and warm it in their hands for at least two minutes.
The canister contains a pressurized liquid. Do not expose to temperatures above 50°C. Do not open, puncture, or burn the canister, even when empty.
Medicines should not be disposed of via wastewater or household waste. Ask a pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Ipravent Inhaler contains
- The active substance of the medicine is ipratropium bromide. One dose contains 20 micrograms of ipratropium bromide (as a monohydrate). Each canister contains 200 doses.
- The other ingredients are: HFC 134a propellant (norflurane), anhydrous citric acid, anhydrous ethanol, and purified water.
- This medicine contains fluorinated greenhouse gases.
- Each inhaler contains 12.3 g of HFC-134a (norflurane), which is equivalent to 0.018 tons of CO2 equivalent (GWP = 1430).
What Ipravent Inhaler looks like and what the pack contains
Ipravent Inhaler is a colorless solution in an aluminum canister with a suitable metering valve and a plastic actuator.
The inner packaging is an anodized aluminum canister with a capacity of 19 ml, equipped with a 50-microliter metering valve and a plastic actuator. The valve consists of a thermoplastic, two-part core, body, measuring chamber, 2 elastomeric seats, and a seal, metal sleeve, and spring. The plastic actuator body and mouthpiece cover are made of polypropylene.
Each canister contains 200 measured doses.
Pack sizes
Single pack
Each pack contains one canister containing 200 doses.
Multi-pack
400 doses (2 x 200). A set containing 2 single packs.
600 doses (3 x 200). A set containing 3 single packs.
Not all pack sizes may be marketed.
Marketing authorization holder
Glenmark Pharmaceuticals s.r.o.
Hvězdova 1716/2b
140 78 Prague 4
Czech Republic
Manufacturer/Importer
Cipla Europe NV, De Keyserlei 60C, Bus 1301, 2018, Antwerp, Belgium
Glenmark Pharmaceuticals s.r.o., Fibíchova 143, 566 17 Vysoké Mýto
Czech Republic
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
Sweden
Ipravent
Poland
Ipravent Inhaler
Germany
Ipravent 20 micrograms/spray, pressurized inhalation, solution
For more information, contact the local representative of the marketing authorization holder:
Glenmark Pharmaceuticals Sp. z o. o.
ul. Dziekońskiego 3
00-728 Warsaw
Email: [email protected]
Date of last revision of the leaflet:December 2024
- Country of registration
- Active substance
- Prescription requiredYes
- ImporterCipla Europe N.V. Glenmark Pharmaceuticals s.r.o.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Ipravent InhalerDosage form: Aerosol, 20 mcg/dose inh.Active substance: ipratropium bromideManufacturer: Laboratorio Aldo-Union S.L. Laboratorio Echevarne, S.A.Prescription requiredDosage form: Aerosol, 20 mcg/dose inh.Active substance: ipratropium bromideManufacturer: Boehringer Ingelheim Pharma GmbH & Co. KGPrescription requiredDosage form: Solution, 0.25 mg/mlActive substance: ipratropium bromideManufacturer: Instituto De Angeli S.r.l.Prescription required
Alternatives to Ipravent Inhaler in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Ipravent Inhaler in Ukraine
Alternative to Ipravent Inhaler in Spain
Online doctors for Ipravent Inhaler
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Ipravent Inhaler – subject to medical assessment and local rules.














