
Atrovent N

Ask a doctor about a prescription for Atrovent N

How to use Atrovent N
Package Leaflet: Information for the Patient
ATROVENT N, 20 micrograms/dose, inhalation aerosol, solution
Ipratropium bromide
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist. See section 4.
Contents of the package leaflet:
- 1. What Atrovent N is and what it is used for
- 2. Important information before using Atrovent N
- 3. How to use Atrovent N
- 4. Possible side effects
- 5. How to store Atrovent N
- 6. Contents of the pack and other information
1. What Atrovent N is and what it is used for
Atrovent N is a bronchodilator with anticholinergic action. Atrovent N is indicated as a bronchodilator for maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease (COPD) including chronic bronchitis and emphysema, and for asthma.
2. Important information before using Atrovent N
When not to use Atrovent N
- if you are allergic to atropine or its derivatives (such as the active substance ipratropium bromide) or to any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Before starting treatment with Atrovent N, discuss with your doctor:
- if you have narrow-angle glaucoma (increased pressure in the eye),
- if you have urinary retention,
- if you have cystic fibrosis: gastrointestinal motility disturbances may occur.
You should immediately contact your doctor:
- in case of bronchospasm induced by inhalation: you should immediately stop using Atrovent N, as this may be life-threatening. You should go to your doctor, who will prescribe other treatment.
- in case of eye complications such as eye pain or discomfort, blurred vision, seeing a rainbow around lights or changes in color vision accompanied by eye redness, you should immediately consult your doctor, who will assess whether these symptoms may be due to complications (dilated pupils, increased eye pressure, narrow-angle glaucoma).
- if breathing problems do not improve or worsen: you should contact your doctor, who will verify the treatment plan. Your doctor may consider adding other medications.
Never use a higher dose than prescribed, as this may lead to severe side effects.
- after administration of the medicine, immediate-type hypersensitivity reactions may occur, as confirmed by rare cases of urticaria, angioedema (sudden swelling of the skin or mucous membranes, which may cause difficulty breathing), rash, bronchospasm, swelling of the mucous membrane of the mouth and throat, and rapidly progressing, life-threatening allergic reactions.
Do not let Atrovent N get into your eyes.
Atrovent N and other medicines
Tell your doctor or pharmacist about all medicines you are taking, or have recently taken, and about medicines you plan to take.
Unless your doctor has told you otherwise, you should avoid long-term concomitant use of Atrovent N with other anticholinergic drugs.
Beta-2 adrenergic receptor stimulants and xanthine derivatives (theophylline) may enhance the bronchodilating effect.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before taking this medicine.
Although non-clinical studies have not shown any adverse effects of ipratropium bromide on the unborn child, Atrovent N should only be used during pregnancy if your doctor considers it necessary.
It is not known whether ipratropium bromide passes into breast milk. Therefore, Atrovent N should only be used in breastfeeding women if your doctor considers it necessary.
Driving and using machines
No studies have been conducted on the effects on the ability to drive and use machines. However, during treatment with Atrovent N, side effects such as dizziness, visual disturbances, dilated pupils, and blurred vision may occur.
Therefore, caution is recommended when driving a car or operating machinery.
Atrovent N contains ethanol
This medicine contains 8.415 mg of ethanol (alcohol) in each actuation (in each metered dose).
The amount of alcohol in each actuation of this medicine is equivalent to less than 1 ml of beer or 1 ml of wine.
The small amount of alcohol in this medicine will not produce noticeable effects.
3. How to use Atrovent N
Atrovent N should always be used as directed by your doctor. If you are unsure, consult your doctor or pharmacist.
Your doctor will adjust the dosage to your individual needs. The recommended dosage for adults and children over 6 years is:
Adults and children over 6 years:
2 actuations (inhalations) at each use, 4 times a day, but not more than 12 actuations per day.
Do not exceed the recommended daily dose.
If the treatment does not produce significant improvement, or if the patient's condition worsens, consult your doctor to establish a new treatment plan. In case of sudden or severe shortness of breath (difficulty breathing), contact your doctor immediately.
It is recommended that Atrovent N, inhalation aerosol, be used in children only on the advice of a doctor and under adult supervision.
In case of acute exacerbations of chronic obstructive pulmonary disease, your doctor may prescribe treatment with Atrovent, solution for nebulization.
Method of administration
Before first use of the inhaler, press the dose-release button twice.
During each useof the medicine, follow these steps:
- 1. Remove the protective cap.
- 2. Take a deep breath out.
- 3. Hold the inhaler in the position shown in Fig. 1, with your lips closed around the mouthpiece. The arrow on the canister and the base of the canister should be pointing upwards.
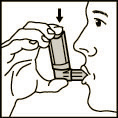
(Fig. 1)
- 4. Take a deep breath in as slowly as possible, while firmly pressing the canister, which will release one metered dose of the medicine. Hold your breath for a few seconds, then remove the mouthpiece from your mouth and breathe out. Repeat these steps for the second inhalation.
- 5. After use, replace the protective cap.
- 6. If the inhaler is not used for 3 days, press the dose-release button once before using it again.
The canister is not transparent. Therefore, you cannot see if it is empty. The inhaler delivers 200actuations. After using this number of doses (usually after 3 weeks of use as directed), a small amount of solution may still be left in the canister. However, you should replace the inhaler with a new one to ensure that each actuation delivers the correct dose of the medicine.
The mouthpiece of the inhaler should be cleaned at least once a week. It is important to keep it clean to ensure that the medicine does not accumulate on the walls of the mouthpiece and block the use of the inhaler.
To clean the mouthpiece of the inhaler, first remove the protective cap and take the canister out of the mouthpiece. Rinse the mouthpiece with warm water until all visible debris is removed.
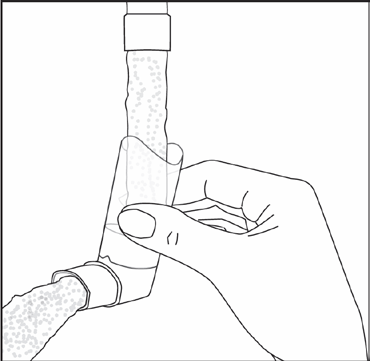
(Fig. 2)
After cleaning, shake the mouthpiece and let it air dry. Do not useany drying devices. When the mouthpiece is dry, put the canister back into the mouthpiece and replace the protective cap.
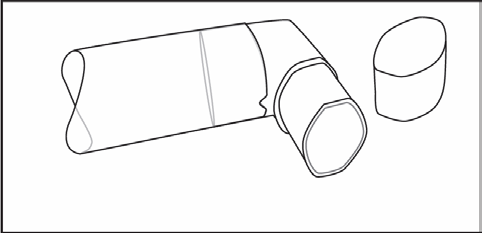
(Fig. 3)
WARNING:
The plastic mouthpiece is designed specifically for use with the Atrovent N inhalation aerosol to ensure that the correct dose of the medicine is delivered each time. The mouthpiece should never be used with any other inhalation aerosol, and Atrovent N inhalation aerosol should never be used with any other mouthpiece.
The canister is under pressure. Do not use force to open it, and do not expose it to temperatures above 50°C.
Overdose of Atrovent N
If you have taken more than the prescribed dose, contact your doctor or pharmacist immediately.
Mild symptoms of ipratropium bromide overdose may occur, such as dryness of the mucous membrane of the mouth, visual disturbances, and increased heart rate.
Missed dose of Atrovent N
If you have been prescribed Atrovent N for regular use and you miss a dose, take it as soon as possible. However, do not take a double dose to make up for the missed dose. Take the next dose at the usual time.
Stopping treatment with Atrovent N
If you stop using Atrovent N, breathing difficulties may recur or worsen. Therefore, you should use Atrovent N for as long as your doctor has prescribed.
In any case, consult your doctor before stopping treatment with Atrovent N.
If you have any further questions about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Atrovent N can cause side effects, although not everybody gets them.
As with all inhaled medicines, Atrovent N may cause local irritation. The most common side effects reported in clinical trials were headache, sore throat, cough, dryness of the mucous membrane of the mouth, gastrointestinal motility disturbances (including constipation, diarrhea, and vomiting), nausea, and dizziness.
The following side effects have been reported during clinical trials with Atrovent N and during post-marketing surveillance.
The frequency of side effects is given as follows:
Very common: occurs in more than 1 in 10 patients
Common: occurs in more than 1 in 100 but less than 1 in 10 patients
Uncommon: occurs in more than 1 in 1,000 but less than 1 in 100 patients
Rare: occurs in more than 1 in 10,000 but less than 1 in 1,000 patients
Very rare: occurs in less than 1 in 10,000 patients
Frequency not known (cannot be estimated from the available data)
Common (occurs in more than 1 in 100 but less than 1 in 10 patients):
- headache,
- dizziness,
- sore throat,
- cough,
- dryness of the mucous membrane of the mouth,
- nausea,
- gastrointestinal motility disturbances.
Uncommon (occurs in more than 1 in 1,000 but less than 1 in 100 patients):
- rapidly progressing, life-threatening allergic reactions (anaphylactic reactions),
- hypersensitivity,
- angioedema (sudden swelling of the skin or mucous membranes, which may cause difficulty breathing),
- palpitations,
- supraventricular tachycardia (abnormally fast heart rate),
- bronchospasm,
- paradoxical bronchospasm (inhalation-induced bronchospasm),
- laryngospasm (sudden constriction of the vocal cords, which may affect breathing and speech),
- oropharyngeal swelling (swelling of the upper part of the throat),
- blurred vision,
- pupil dilation,
- increased intraocular pressure,
- glaucoma,
- eye pain,
- halo vision,
- conjunctival congestion,
- corneal edema (swelling of the protective outer layer of the eye),
- dry throat,
- diarrhea,
- constipation,
- vomiting,
- stomatitis,
- oropharyngeal swelling,
- urinary retention,
- rash,
- pruritus.
Rare (occurs in more than 1 in 10,000 but less than 1 in 1,000 patients):
- atrial fibrillation (very rapid, irregular heart rate),
- increased heart rate,
- accommodation disturbances (visual disturbances),
- urticaria.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, tell your doctor or pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety Monitoring, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: 22 49-21-301, fax: 22 49-21-309, website: https://smz.ezdrowie.gov.pl.
Side effects can also be reported to the marketing authorization holder or its representative. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Atrovent N
Store below 25°C.
Do not freeze.
Protect from direct sunlight.
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the label and carton after EXP.
The expiry date refers to the last day of the month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Atrovent N contains
- The active substance is ipratropium bromide. One metered dose (actuation) contains 21 micrograms of ipratropium bromide (Ipratropii bromidum), equivalent to 20 micrograms of anhydrous ipratropium bromide.
- The other ingredients are HFA 134a (1,1,1,2-tetrafluoroethane), anhydrous citric acid, anhydrous ethanol, purified water.
This medicine contains fluorinated greenhouse gases.
Each inhaler contains 12.33 g of HFA 134a (1,1,1,2-tetrafluoroethane), which is equivalent to 0.01763 t CO2 equivalent (GWP = 1430).
What Atrovent N looks like and contents of the pack
1 canister containing 10 ml solution (200 doses).
Marketing authorization holder and manufacturer
Marketing authorization holder:
Boehringer Ingelheim International GmbH
Binger Strasse 173
D-55216 Ingelheim/Rhein
Germany
Manufacturer:
Boehringer Ingelheim Pharma GmbH & Co. KG
Binger Strasse 173
D-55216 Ingelheim/Rhein
Germany
To obtain more detailed information, contact the representative of the marketing authorization holder:
Poland
Boehringer Ingelheim Sp. z o.o.
Tel.: +48 22 699 0 699
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterBoehringer Ingelheim Pharma GmbH & Co. KG
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Atrovent NDosage form: Aerosol, 20 mcg/dose inh.Active substance: ipratropium bromideManufacturer: Laboratorio Aldo-Union S.L. Laboratorio Echevarne, S.A.Prescription requiredDosage form: Solution, 0.25 mg/mlActive substance: ipratropium bromideManufacturer: Instituto De Angeli S.r.l.Prescription requiredDosage form: Aerosol, 20 mcg/inhalation doseActive substance: ipratropium bromidePrescription required
Alternatives to Atrovent N in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Atrovent N in Ukraine
Alternative to Atrovent N in Spain
Online doctors for Atrovent N
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Atrovent N – subject to medical assessment and local rules.














