
SOFTACORT 3.35 mg/ml EYE DROPS IN SINGLE-DOSE CONTAINERS


How to use SOFTACORT 3.35 mg/ml EYE DROPS IN SINGLE-DOSE CONTAINERS
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet:information for the user
Softacort 3.35 mg/ml eye drops, solution in single-dose containers
Sodium hydrocortisone phosphate
Read the entire package leaflet carefully before starting to use this medication,as it contains important information for you.
- Keep this package leaflet, as you may need to read it again.
- If you have any questions, consult your doctor, pharmacist, or nurse.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are side effects not listed in this package leaflet. See section 4.
Contents of the package leaflet:
- What is Softacort and what is it used for
- What you need to know before taking Softacort
- How to use Softacort
- Possible side effects
- Storage of Softacort
- Container contents and additional information
1. What is Softacort and what is it used for
This medication is an eye drop solution in single-dose containers containing a substance called hydrocortisone. This substance is a corticosteroid that inhibits inflammation symptoms.
It is used to treat mild allergic or inflammatory conditions of the superficial part of your eye or eyes (conjunctiva).
The eye should not be infected (see Do not use Softacort).
2. What you need to know before taking Softacort
Do not use Softacort
- If you are allergic to the active ingredient (hydrocortisone) or to any of the other components of this medication (listed in section 6).
- If you have high eye pressure (ocular hypertension), which is known to be associated with the use of glucocorticoids (corticosteroid family) or other causes.
- If you have acute viral infections caused by the herpes virus and in most other viral diseases in the ulceration phase (unless the infection is being treated with anti-infectious treatment for the herpes virus).
- If you have conjunctivitis with ulcerative inflammation of the cornea (keratitis), even in the initial phase.
- If you have a bacterial infection in the eye (acute purulent infection, conjunctivitis, blepharitis, and hordeolum).
- If you have a fungal infection in the eye (ocular mycosis).
- If you have a bacterial infection called tuberculosis that affects your eye (ocular tuberculosis).
Warnings and precautions
- Consult your doctor, pharmacist, or nurse before starting to use this medication.
- If you have a red eye that has not been diagnosed, do not use this medication.
- If you have a viral eye infection (herpes), use this medication only if the infection is being treated with anti-infectious treatment and close monitoring of your eyes is required.
- If you have a disease that causes thinning of the outer part of your eye (cornea and sclera), there may be a higher risk of perforation due to the use of topical corticosteroids applied to the eye.
- If you have been using a corticosteroid medication for a long time and have an eye wound (corneal ulcer), a fungal infection may be suspected.
- During your treatment, close and regular monitoring of your eyes is required. Prolonged use of corticosteroids has been shown to cause increased pressure inside the eye and the onset of glaucoma, especially in patients who already suffer from high intraocular pressure or are at risk of developing such a condition with local steroid treatment (see Possible side effects) and cause lens opacity in your eye (cataract), particularly in children and the elderly population.
- The use of corticosteroids can cause opportunistic eye infections. Additionally, topical ocular corticosteroids can promote, exacerbate, or mask signs and symptoms of opportunistic eye infections.
- You should avoid wearing contact lenses during treatment with this medication.
- Contact your doctor if you experience blurred vision or other visual disturbances.
Children
There are no safety and efficacy data in children.
Long-term treatment with corticosteroids can produce adrenal suppression.
Increased eye pressure in children occurs more frequently, with greater severity, and with greater rapidity than in adults.
Using Softacort with other medications
Tell your doctor or pharmacist if you are using, have recently used, or may need to use any other medication, including medications that can be obtained without a prescription.
Some medications may increase the effects of Softacort, and your doctor may want to monitor you closely if you are using these medications (including some medications for HIV: ritonavir, cobicistat).
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
- The use of this medication is not recommended during pregnancy except when your doctor considers it necessary and under strict supervision.
It is unknown whether this medication passes into breast milk. Your doctor will decide whether you can use this medication during breastfeeding.
Driving and using machines
You may experience blurred vision immediately after applying Softacort. Do not drive or use machines until this effect has disappeared.
Softacort contains phosphate
This medication contains 0.227 mg of phosphates in each drop.
3. How to use Softacort
Follow the administration instructions for this medication exactly as indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again.
The recommended dose is 2 drops in the affected eye(s), 2 to 4 times a day, as prescribed by your doctor. A gradual reduction in dose is recommended to avoid relapse. The duration of treatment may vary from a few days to a maximum of 14 days.
The same dose is used for both adults and elderly patients.
Use in children
The efficacy and safety have not been established in children.
Instructions for use
This medication is intended for administration in the eye.
To use the eye drops, follow these instructions:
- Wash your hands and sit or stand comfortably.
- Open the pouch containing 10 single-dose containers. Note the date of first opening on the pouch.
- Separate a single-dose container from the strip.
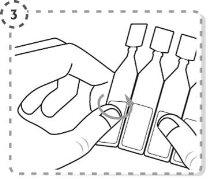
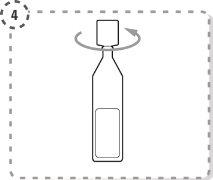
- Turn the tip of the single-dose container as shown. Do not touch the tip after opening the container.
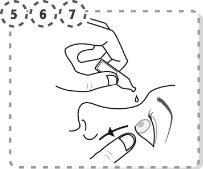
- Use your finger to gently separate the lower eyelid of the affected eye.
- Place the tip of the single-dose container near the eye, without touching it.
- Gently press the single-dose container so that two drops fall into the eye, then remove your finger from the lower eyelid.
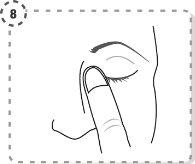
- Press the tip of the affected eye, near the nose, with your finger. Apply pressure for 1 minute, keeping your eye closed.
- Repeat the operation in the other eye, if your doctor has indicated it. Each single-dose container contains enough for both eyes.
- Discard the single-dose container after use. Do not save it for later use.
- Place the unopened single-dose containers inside the pouch. Place the opened pouch inside the box. Unopened containers can be used within one month after opening the pouch.
If you use any other eye drops, wait at least 5 minutes between each application.
If you use more Softacort than you should
Rinse your eye with sterile water if too much product has been administered in the eye and there is prolonged irritation.
Contact your doctor or pharmacist immediately.
If you forget to use Softacort
Do not apply an additional drop in the eye to make up for the missed dose.
If you stop treatment with Softacort
Do not stop treatment suddenly. Always consult your doctor if you are considering stopping treatment.
If you have any other questions about using this medication, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone gets them.
Frequency not known:cannot be estimated from available data
- transient eye discomfort (burning, stinging) after application.
The following side effects have been reported with medications of the same group (corticosteroids) when used to treat eye conditions.
Frequency unknown:cannot be estimated from available data
- allergic reactions
- delayed wound healing
- lens opacity (posterior subcapsular cataract)
- opportunistic infections (herpes-like viral, fungal)
- increased pressure in your eye (glaucoma)
- pupil dilation (mydriasis)
- drooping eyelids (ptosis)
- inflammation inside your eye (uveitis)
- changes in the thickness of the front part of your eye (cornea)
- corneal inflammation (crystalline keratopathy)
- blurred vision
If you suffer from severe damage to the transparent layer of the front part of your eye (cornea), treatment with phosphates, in very rare cases, can cause cloudy patches on the cornea due to calcium.
Reporting side effects
If you experience any side effects, consult your doctor, pharmacist, or nurse, even if they are side effects not listed in this package leaflet. You can also report them directly through the Spanish Medication Surveillance System for Human Use: https//: notificaram.es. By reporting side effects, you can contribute to providing more information on the safety of this medication.
5. Storage of Softacort
Keep this medication out of sight and reach of children.
Do not use this medication after the expiration date shown on the box, pouch, and single-dose container after CAD or EXP. The expiration date is the last day of the month indicated.
Store below 25°C.
After first opening the pouch:use the single-dose container within one month.
Keep the single-dose containers inside the pouch to protect them from light.
Write the date of first opening of the pouch.
After first opening the single-dose container:use immediately and discard the single-dose container after use.
Sterility cannot be maintained after opening a single-dose container, so you must open a new container before each use.
Medications should not be disposed of through wastewater or household waste. Deposit the containers and medications you no longer need at the SIGRE collection point in your pharmacy. If in doubt, ask your pharmacist how to dispose of containers and medications you no longer need. This will help protect the environment.
6. Container contents and additional information
Composition ofSoftacort
- The active ingredient is sodium hydrocortisone phosphate.
1 ml of eye drop solution contains 3.35 mg of sodium hydrocortisone phosphate.
- The other components are disodium phosphate dodecahydrate, sodium dihydrogen phosphate monohydrate, sodium chloride, disodium edetate, hydrochloric acid (for pH adjustment), and water for injectable preparations.
Appearance of the product and container contents
This medication is presented as an eye drop solution in single-dose containers.
The solution is practically transparent, colorless to slightly yellow, practically free of particles, presented inside a pouch with 10 units, each single-dose container contains 0.4 ml of eye drop solution.
The boxes contain 10 (1 x 10), 20 (2 x 10), 30 (3 x 10), or 60 (6 x 10) single-dose containers.
Only some package sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder
LABORATOIRES THEA
12 RUE LOUIS BLERIOT
63017 CLERMONT-FERRAND CEDEX 2
FRANCE
Manufacturer
LABORATOIRES UNITHER
1 rue de l’Arquerie
50200 Coutances
FRANCE
or
LABORATOIRES THEA
12, rue Louis Blériot
63017 CLERMONT-FERRAND Cedex 2
FRANCE
Local representative
LABORATORIOS THEA, S.A.
C/ Enric Granados nº 86-88, 2ª planta
08008 – BARCELONA
This medication is authorized in the Member States of the European Economic Area under the following names:
Germany, Austria, Bulgaria, Cyprus, Croatia, Denmark, Slovenia, Spain, Finland, France, Greece, Iceland, Norway, Poland, Portugal, Czech Republic, Slovak Republic, United Kingdom, Romania, Sweden Softacort
Belgium, Netherlands, Luxembourg Softacor
Ireland Zoftazot
Italy Sofacor
Date of last revision of this package leaflet: October 2023
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SOFTACORT 3.35 mg/ml EYE DROPS IN SINGLE-DOSE CONTAINERSDosage form: OPHTHALMIC OINTMENT, 15 mg/gActive substance: hydrocortisoneManufacturer: Omnivision GmbhPrescription requiredDosage form: EYEDROP, 1 mg/mlActive substance: dexamethasoneManufacturer: Pharmaselect International Beteiligungs GmbhPrescription requiredDosage form: EYE DROP, 0.1 %Active substance: dexamethasoneManufacturer: Fidia Farmaceutici S.P.A.Prescription required
Online doctors for SOFTACORT 3.35 mg/ml EYE DROPS IN SINGLE-DOSE CONTAINERS
Discuss questions about SOFTACORT 3.35 mg/ml EYE DROPS IN SINGLE-DOSE CONTAINERS, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions










