
Tobramicina Sun
Ask a doctor about a prescription for Tobramicina Sun

How to use Tobramicina Sun
Package Leaflet: Information for the Patient
Tobramycin SUN, 300 mg/5 mL, Solution for Nebulisation
tobramycin
Read the package leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- Ask your doctor or pharmacist if you have any further questions.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, tell your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the Package Leaflet:
- 1. What Tobramycin SUN is and what it is used for
- 2. Important information before using Tobramycin SUN
- 3. How to use Tobramycin SUN
- 4. Possible side effects
- 5. How to store Tobramycin SUN
- 6. Contents of the pack and other information
1. What Tobramycin SUN is and what it is used for
Tobramycin SUN contains the antibiotic tobramycin. It belongs to a group of antibiotics called aminoglycosides.
This medicine is used in patients aged 6 years and older with cystic fibrosis to treat lung infection caused by the bacterium Pseudomonas aeruginosa.
Tobramycin SUN fights the infection in the lungs caused by Pseudomonasbacteria and makes breathing easier.
After inhaling this antibiotic, it goes directly to the lungs to fight the bacteria causing the infection. You should follow the instructions in this leaflet to get the best results.
What is Pseudomonas aeruginosa?
It is a very common bacterium that causes lung infections in some period of life in almost all patients with cystic fibrosis. In some people, this infection occurs only at an advanced age, while in others it appears in early childhood.
It is one of the most dangerous bacteria for people with cystic fibrosis. Untreated lung infections caused by this bacterium lead to further lung damage and thus cause breathing problems.
Tobramycin SUN works by killing the bacteria that cause lung infections. Infections can be successfully controlled if they are detected early.
2. Important information before using Tobramycin SUN
When NOT to use Tobramycin SUN
- if you are allergic to tobramycin, any other aminoglycoside antibiotic, or any other ingredient of this medicine (listed in section 6).
If any of the above applies to you, do not use this medicine and consult your doctor.
If you think you may be allergic, consult your doctor.
Warnings and precautions
Before starting to use Tobramycin SUN, you should discuss with your doctor if you have or have ever had any of the following:
- hearing problems (including ringing in the ears and dizziness)
- kidney disease
- breathing difficulties with wheezing or coughing, chest tightness
- blood in sputum (coughed-up mucus)
- muscle weakness, persistent or worsening over time, such as in conditions like myasthenia (muscle weakness) or Parkinson's disease. If any of the above applies to you, you should inform your doctor before using Tobramycin SUN.
Inhalation medicines can cause chest tightness and wheezing, and this may also happen with Tobramycin SUN. Your doctor will supervise the administration of the first dose of the medicine and check your lung function before and after it is given. If you are not using a bronchodilator (such as salbutamol), your doctor may prescribe one before using Tobramycin SUN.
Over time, while using Tobramycin SUN, the Pseudomonasbacteria may become resistant to the medicine. This means that the medicine may not be as effective when used for a long time. If you have any doubts, consult your doctor.
In patients receiving tobramycin by injection, it can lead to hearing loss, dizziness, and kidney damage, and in pregnant women to fetal damage.
Children and adolescents
Tobramycin SUN can be used in adolescents and children aged 6 years and older. This medicine should not be given to children under 6 years of age.
Older people
In patients aged 65 years and older, before deciding to use this medicine, your doctor may recommend additional tests.
Tobramycin SUN and other medicines
Tell your doctor or pharmacist about all medicines you are taking or have recently taken, and about any medicines you plan to take.
DO NOTuse the following medicines while being treated with Tobramycin SUN:
- diuretics such as furosemide or ethacrynic acid
- other medicines that may affect kidney function, such as urea or mannitol given by injection
- other medicines that may harm the nervous system, kidneys, or hearing.
The following medicines may increase the risk of side effects if taken while receiving tobramycin injections:
- amphotericin B, cephalothin, cyclosporin, polymyxins (used to treat bacterial infections), tacrolimus (used to weaken the immune system). These medicines may harm the kidneys.
- platinum compounds such as carboplatin and cisplatin (used to treat certain types of cancer). These medicines may harm the kidneys or hearing.
- cholinesterase inhibitors such as neostigmine and pyridostigmine (used to treat muscle weakness) or botulinum toxin. These medicines may cause or worsen muscle weakness.
If you are taking any of the above medicines, consult your doctor before using Tobramycin SUN.
Do not mix Tobramycin SUN with other medicines in the nebulizer.
If you are using several different treatments for cystic fibrosis, you should use them in the following order:
- 1. administer a bronchodilator (such as salbutamol);
- 2. perform chest physiotherapy;
- 3. administer other inhalation medicines;
- 4. then use Tobramycin SUN. Consult your doctor about the above order.
Pregnancy and breastfeeding
Pregnancy
If you are pregnant, think you may be pregnant, or plan to have a baby, ask your doctor for advice before using this medicine.
It is not known whether inhalation of the medicine during pregnancy causes side effects. Tobramycin and other aminoglycoside antibiotics given by injection during pregnancy may harm the fetus, causing, for example, deafness.
Breastfeeding
If you are breastfeeding, ask your doctor for advice before using this medicine.
Driving and using machines
It is unlikely that this medicine will affect your ability to drive or use machines.
3. How to use Tobramycin SUN
Always use this medicine exactly as your doctor has told you. If you are not sure, ask your doctor.
It is recommended to use twoampoules per day (one in the morning and one in the evening) for 28 days.
- the dose is the same for all people aged 6 years and older;
- the contents of one ampoule should be inhaled through the mouth using a nebulizer (one ampoule in the morning and one in the evening);
- it is best to keep the interval between doses as close to 12 hours as possible. This interval must be at least 6 hours;
- after 28 days of using the medicine, there is a 28-day break during which you should not take Tobramycin SUN by inhalation. After the break, you should start the next treatment (according to the scheme).
- it is important to use the medicine twice a day for 28 days and follow the cycle: 28 days of treatment, followed by 28 days of rest;
Using Tobramycin SUN Break in using Tobramycin SUN
Tobramycin SUN
Use twice a day,
every day for 28 days
Do not use the medicine for the next 28 days
repeat the cycle
Continue using the medicine in the above way for as long as your doctor recommends. If you have any doubts about the duration of treatment with Tobramycin SUN, ask your doctor or pharmacist.
Instructions for using Tobramycin SUN
This part of the leaflet explains how to use, care for, and handle Tobramycin SUN.
Read this instruction carefully and follow it.
If you have any further doubts about using this medicine, ask your doctor or pharmacist.
Equipment required for Tobramycin SUN inhalation
The medicine should be used with a clean and dry reusable nebulizer.
The LC PLUS nebulizer (manufactured by PARI GmbH) is suitable for use with this medicine.
Your doctor or physiotherapist can give you advice on the proper use of the medicine with the required equipment. Different nebulizers may be needed to administer other inhalation medicines used in cystic fibrosis.
Preparing Tobramycin SUN for inhalation
- Wash your hands thoroughly with water and soap.
- Each foil bag contains 4 ampoules. Tear or cut the bag. Remove one ampoule of the medicine from the foil bag. Store the remaining ampoules in the refrigerator in the original packaging.
- Place all parts of the nebulizer on a clean, dry paper or towel.
- Make sure you have the correct compressor and tube to connect the nebulizer.
- Follow the instructions for the proper use of the nebulizer, and read the manufacturer's leaflet. Before using the medicine, make sure the nebulizer and compressor are working properly according to the manufacturer's instructions.
Using Tobramycin SUN with the LC PLUS nebulizer (PARI GmbH)
To get more detailed instructions for the proper use of the nebulizer, read the leaflet that comes with the PARI LC PLUS model.
- 1. Remove the nebulizer cover (turn it counterclockwise and lift it). Place the cover on a towel and put the nebulizer chamber on it in a vertical position.
- 2. Connect one end of the tube to the air outlet of the compressor. Make sure the tube fits tightly to the outlet. Connect the compressor to the power outlet.
- 3. Open the Tobramycin SUN ampoule by holding the lower part in one hand and twisting the upper part with the other hand. Squeeze the entire contents of the ampoule into the nebulizer chamber.
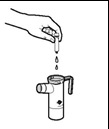
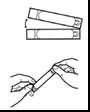
- 4. Replace the nebulizer cover, put the mouthpiece and inhalation valve cover in place on the nebulizer, and connect the compressor according to the instructions in the PARI LC PLUS nebulizer leaflet.
- 5. Turn on the compressor. Check if a continuous mist is coming out of the mouthpiece. If not, check all tube connections and make sure the compressor is working properly.
- 6. Stand or sit upright so you can breathe normally.
- 7. Put the mouthpiece between your teeth and the tip of your tongue. Breathe normally, but only through your mouth (if your doctor agrees, you can use a nose clip). Do not block the airflow with your tongue.
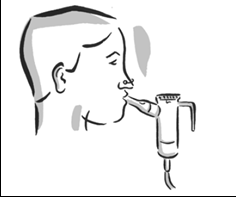
- 8. Continue until you have taken the entire dose of Tobramycin SUN and the mist has disappeared. Taking the entire dose takes about 15 minutes. After the nebulizer is empty, it may make a gurgling sound.
- 9. After use, remember to clean and disinfect the nebulizer according to the manufacturer's instructions. Do not use a dirty or clogged nebulizer. Do not lend the nebulizer to others.
If you need to interrupt or cough during treatment, turn off the compressor to save the medicine. Turn it back on when you are ready to take the medicine again.
Missed doses should be skipped if the next dose is due in less than 6 hours.
Using more Tobramycin SUN than recommended
If you inhale more of the medicine than recommended, you may experience hoarseness.
Tell your doctor as soon as possible.
If you swallow the medicine, tell your doctor as soon as possible.
Missing a dose of Tobramycin SUN
If you miss a dose of Tobramycin SUN, and it is at least 6 hours before the next dose, take the dose as soon as possible. Otherwise, wait until it is time for the next dose. Do not take a double dose to make up for a missed dose.
Stopping Tobramycin SUN treatment
Do not stop using the medicine unless your doctor tells you to, as this may lead to inadequate control of the lung infection and its worsening.
If you have any further doubts about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Severe side effects
If you experience any of the following severe side effects, tell your doctor immediately.
- unusual breathing difficulties with wheezing or coughing and chest tightness
- allergic reactions including hives and itching
If you experience any of the following severe side effects, tell your doctor immediately:
- hearing loss (ringing in the ears that may be a sign of hearing loss), ear noises (such as buzzing)
While using Tobramycin SUN, the underlying lung disease may worsen. This may be because the medicine did not work. Tell your doctor immediately if this happens.
Other side effects
Tell your doctor as soon as possible if you experience any of the following side effects:
Very common (may affect more than 1 in 10 people)
- runny nose or nasal congestion, sneezing
- voice change (hoarseness)
- change in the color of sputum (mucus)
- worsening of lung function test results
Common (may affect up to 1 in 10 people)
- general feeling of being unwell
- muscle pain
- voice change with sore throat and difficulty swallowing (laryngitis)
Other side effects
- itching
- itchy rash
- rash
- loss of voice
- taste disturbances
- sore throat
Unknown (frequency cannot be estimated from available data)
- increased amount of sputum (mucus)
- chest pain
- decreased appetite
When using Tobramycin SUN at the same time as or after multiple cycles of tobramycin or other aminoglycoside antibiotics by injection, hearing loss has been reported as a side effect.
Tobramycin or other aminoglycoside antibiotics by injection may cause allergic reactions, hearing disturbances, or kidney damage.
In people with cystic fibrosis, many symptoms of the disease may occur. They may continue to occur during treatment with Tobramycin SUN, but they should not occur more frequently or be more severe than before.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, tell your doctor or pharmacist, or nurse. Side effects can be reported directly to the Department of Drug Safety, Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products:
Jerozolimskie Avenue 181C, 02-222 Warsaw, phone: 22 49-21-301, fax: 22 49-21-309. Website: https://smz.ezdrowie.gov.pl.
Side effects can also be reported to the marketing authorization holder.
Reporting side effects will help to gather more information on the safety of this medicine.
5. How to store Tobramycin SUN
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the packaging after EXP.
The expiry date refers to the last day of the month stated.
Store in a refrigerator (2 - 8°C). Store in the original packaging to protect from light.
During transport, the medicine can be stored in bags (unopened or opened) at a temperature below 25°C for up to 28 days. Do not use the medicine if it has been stored at room temperature for more than 28 days.
The medicine is colorless to light yellow, but this is not a rule, and its color may be slightly darker. This does not affect the medicine's action if it has been stored properly.
Do not use the medicine if it becomes cloudy or if there are solid particles in the solution.
Never store an opened ampoule. Once opened, the ampoule should be used immediately, and any remaining medicine should be discarded.
The ampoules are labeled in English with "For oral inhalation only" and "STERILE", which means "Only for inhalation" and "STERILE", respectively.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Tobramycin SUN contains
- The active substance is tobramycin. Each 5 mL ampoule contains 300 mg of tobramycin, which corresponds to 60 mg/mL.
- The other ingredients are sodium chloride, water for injections, nitrogen (E941), sulfuric acid (E513) (for pH adjustment), and/or sodium hydroxide (E524) (for pH adjustment).
What Tobramycin SUN looks like and contents of the pack
Clear, colorless, sterile solution for injection, which does not contain visible solid particles.
The medicine is available in a ready-to-use ampoule. The ampoules are packed in foil bags of 4, which is enough for 2 days of treatment.
The medicine is available in packs of 56, 112, or 168 ampoules, which are sufficient for one, two, or three treatment cycles, respectively.
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Sun Pharmaceutical Industries Europe B.V.
Polarisavenue 87
2132 JH Hoofddorp
Netherlands
Manufacturer/Importer
Sun Pharmaceutical Industries Europe B.V.
Polarisavenue 87
2132 JH Hoofddorp
Netherlands
Terapia S.A.
124 Fabricii Street
400632, Cluj-Napoca
Cluj County
Romania
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Germany:
Tobramycin SUN 300 mg Lösung für einen Vernebler
Denmark:
Tobramycin SUN
Spain:
Tobramicina SUN 300 mg/ 5 ml solución para inhalación por nebulizador
France:
Tobramycine SUN 300 mg/ 5 ml solution pour inhalation par nébuliseur
Italy:
Tobramicina SUN
Netherlands:
Tobramycine SUN 300 mg/ 5 ml verneveloplossing
Romania:
Tobramicină SUN 300 mg soluţie pentru nebulizator
United Kingdom (Northern Ireland):
Tobramycin 300 mg/ 5 ml nebuliser solution
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterSun Pharmaceutical Industries Europe B.V. Terapia S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Tobramicina SunDosage form: Solution, 300 mg/4 mlActive substance: tobramycinManufacturer: Chiesi Farmaceutici S.p.A. Genetic S.p.APrescription requiredDosage form: Solution, 1 mg/mlActive substance: tobramycinManufacturer: B. Braun Medical S.A.Prescription requiredDosage form: Solution, 3 mg/mlActive substance: tobramycinManufacturer: B. Braun Medical S.A.Prescription required
Alternatives to Tobramicina Sun in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Tobramicina Sun in Ukraine
Alternative to Tobramicina Sun in Spain
Online doctors for Tobramicina Sun
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Tobramicina Sun – subject to medical assessment and local rules.














