
TOBRAMICIN TEVA 300 mg/5 ml SOLUTION FOR NEBULIZER INHALATION

How to use TOBRAMICIN TEVA 300 mg/5 ml SOLUTION FOR NEBULIZER INHALATION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
- Introduction
- What Tobramycin Teva Solution for Nebulisation is and what it is used for
- What you need to know before you use Tobramycin Teva Solution for Nebulisation
- How to use Tobramycin Teva Solution for Nebulisation
- Possible Adverse Effects
- Storage of Tobramycin Teva Nebulizer Solution
- Package Contents and Additional Information
Introduction
Package Leaflet: Information for the User
Tobramycin Teva 300 mg/5 ml Solution for Nebulisation
Read all of this leaflet carefully before you start using this medicine because it contains important information for you.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
- If you get any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this leaflet. See section 4.
Contents of the pack and other information:
- What Tobramycin Teva Solution for Nebulisation is and what it is used for
- What you need to know before you use Tobramycin Teva Solution for Nebulisation
- How to use Tobramycin Teva Solution for Nebulisation
- Possible side effects
5 Storage of Tobramycin Teva Solution for Nebulisation
- Contents of the pack and further information
1. What Tobramycin Teva Solution for Nebulisation is and what it is used for
Tobramycin Teva Solution for Nebulisation contains the active substance tobramycin. This is an antibiotic belonging to the aminoglycoside family.
Antibiotics are used to treat bacterial infections and are not effective against viral infections such as the flu or the common cold. It is important that you follow the instructions regarding dose, administration interval, and treatment duration as indicated by your doctor. Do not store or reuse this medicine. If you have any leftover antibiotic after completing your treatment, return it to the pharmacy for proper disposal. Do not dispose of medicines via wastewater or household waste. |
Tobramycin Teva Solution for Nebulisation is used in patients aged 6 years and older who have cystic fibrosis, for the treatment of lung infections caused by the bacterium Pseudomonas aeruginosa.
Tobramycin fights the infection caused by the Pseudomonasbacteria in your lungs and helps improve your breathing.
When you inhale tobramycin, the antibiotic can go directly to your lungs to fight the bacteria causing the infection. For the best results with this medicine, follow the instructions in this leaflet.
What is Pseudomonas aeruginosa?
It is a very common bacterium that infects almost all patients with cystic fibrosis at some point in their lives. Some of them do not get this infection until very late in life, while others get it at a very young age.
This is one of the most harmful bacteria for people with cystic fibrosis. If the infection is not properly controlled, it can continue to damage your lungs, causing additional breathing problems.
Tobramycin kills the bacteria that cause lung infections. This infection can be successfully controlled if the problem is addressed at an early stage.
2. What you need to know before you use Tobramycin Teva Solution for Nebulisation
Do not use Tobramycin Teva Solution for Nebulisation
- if you are allergic to tobramycin, any other aminoglycoside antibiotic, or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Consult your doctor before starting to use Tobramycin Teva Solution for Nebulisation if you have or have ever had any of the following conditions:
- Hearing problems (including ringing in the ears and dizziness).
- Kidney problems
- Unusual difficulty breathing with wheezing or coughing, chest tightness
- Blood in your sputum (the matter you cough up)
- Muscle weakness that lasts or worsens over time, symptoms mostly related to myasthenia or Parkinson's disease.
If any of these cases apply to you, inform your doctor before using Tobi.
Inhaling medicines can cause chest tightness and wheezing, and this can occur with Tobi. Your doctor will supervise your first dose of Tobi and check your lung function before and after the dose. If you are not doing so, your doctor may have you use a bronchodilator (e.g., salbutamol) before using Tobi.
If you are using Tobi, Pseudomonasstrains may become resistant to treatment over time. This means that over time, the medicine may not work as well as it should. Consult your doctor if you are concerned about this issue.
If administration is by injection, tobramycin can occasionally cause hair loss, dizziness, and kidney damage and may harm the fetus.
Children and adolescents
Tobramycin Solution for Nebulisation can be administered to children and adolescents aged 6 years and older. Tobramycin cannot be administered to children under 6 years of age.
Elderly
If you are 65 years of age or older, your doctor may perform additional tests to decide if Tobramycin Solution for Nebulisation is a suitable treatment for you.
Using Tobramycin Teva Solution for Nebulisation with other medicines
Tell your doctor or pharmacist if you are using, have recently used, or might use any other medicines, including those obtained without a prescription.
Do not take the following medicines while using Tobramycin Teva Solution for Nebulisation:
- Furosemide or ethacrynic acid, diuretics.
- Intravenous urea or mannitol.
- Other medicines that can harm your nervous system, kidneys, or ears.
The following medicines may increase the risk of harmful effects if administered while you are receiving tobramycin injections:
- Amphotericin B, cephalothin, cyclosporine, tacrolimus, polymyxins: these medicines can harm your kidneys.
- Platinum compounds (such as carboplatin and cisplatin): these medicines can harm your kidneys or ears.
- Anticholinesterases (such as neostigmine and pyridostigmine) or botulinum toxin: these medicines can cause or worsen muscle weakness.
If you are taking one or more of the above medicines, discuss this with your doctor before using Tobramycin Solution for Nebulisation.
Do not mix or dilute Tobramycin Solution for Nebulisation with any other medicine in your nebuliser.
If you are taking several different treatments for cystic fibrosis, you should take them in the following order:
- bronchodilator treatment, such as salbutamol
- chest physiotherapy
- other medicines for inhalation
- Tobi last.
Also, check this order with your doctor.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Pregnancy
If you are pregnant or plan to become pregnant, consult your doctor about the possibility that this medicine may harm you or your fetus.
It is not known if inhaling this medicine during pregnancy causes harmful effects. When administered by injection, tobramycin and other aminoglycoside antibiotics can cause harm to the fetus, such as deafness.
Breastfeeding
It is not known if inhaled tobramycin can be detected in breast milk at the recommended dose. If you are breastfeeding, consult your doctor before using any medicine.
Driving and using machines
This medicine is not expected to affect your ability to drive or use machines.
3. How to use Tobramycin Teva Solution for Nebulisation
For inhalation use only.
Follow exactly the administration instructions for this medicine as indicated by your doctor or pharmacist. If you are unsure, ask your doctor or pharmacist.
How much to use and how often to use it
- The recommended dose is the same for all patients aged 6 years and older.
- Use twoampoules each day, for 28 days. Inhale the contents of one ampoule in the morning and one in the evening. Ideally, there should be an interval of 12 hours between doses.
- You must leave a minimum interval of 6 hoursbetween the two inhalations of tobramycin.
- After taking your medicine for 28 days, you will then have a 28-day period during which you should not inhale any dose of tobramycin, before starting another cycle.
- It is important that you maintain the use of the product twice daily during your 28-day treatment period and maintain the 28-day treatment cycles, 28-day rest cycles.
ON Tobramycin Solution for Nebulisation | OFF Tobramycin Solution for Nebulisation |
Take tobramycin solution for nebulisation twice a day. Each day for 28 days. | Do not take tobramycin solution for nebulisation during the next 28 days. |
Repeat the cycle
If you use more Tobramycin Solution for Nebulisation than you should
If you inhale too much tobramycin, your voice may become very hoarse. Make sure to inform your doctor as soon as possible. If you swallow tobramycin solution for nebulisation, inform your doctor as soon as possible. You can also immediately consult your pharmacist or call the Toxicology Information Service, phone 91 562 04 20, indicating the medicine and the amount ingested or inhaled.
If you forget to use Tobramycin Solution for Nebulisation
If you forget to use Tobramycin Solution for Nebulisation and it is at least 6 hours before your next dose, take a new dose as soon as possible. Otherwise, wait for your next dose. Do not take a double dose to make up for forgotten doses.
If you stop using Tobramycin Solution for Nebulisation
Do not stop using Tobramycin Solution for Nebulisation until you have completed your treatment or your doctor has told you to do so.
Instructions for using Tobramycin Solution for Nebulisation
This part of the leaflet explains how to use, care for, and handle tobramycin solution for nebulisation. Read and follow these instructions carefully.
The equipment you need to inhale Tobramycin Teva Solution for Nebulisation
Tobramycin Teva Solution for Nebulisation should be used with a clean and dry, reusable PARI LC PLUS nebuliser, along with a suitable compressor. Ask your doctor or physiotherapist for information about which compressor is best for you. Your doctor or physiotherapist can advise you on the proper use of your medicine and the equipment you need. You may need different nebulisers for other inhaled medicines for cystic fibrosis (CF).
Before starting treatment with Tobramycin Teva Solution for Nebulisation, make sure you have the following equipment:
- Tobramycin ampoule
- Reusable PARI LC PLUS nebuliser
- Compressor available
- Tube to connect the nebuliser and compressor
- Clean paper or a cloth towel
- Nose clips (if necessary)
Before using your medicine, check that your nebuliser and compressor are working correctly according to the manufacturer's instructions.
Instructions for use:
- Wash your hands well with water and soap.
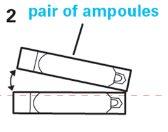
- Each bag contains 4 ampoules. Cut or tear the bag, remove one ampoule, and store the bag in the refrigerator.
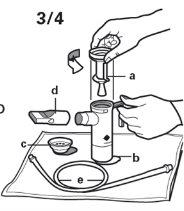
- Place the parts of your PARI LC PLUS nebuliser on a clean, dry paper or a cloth towel. You should have the following elements:
- Nebuliser top
- Nebuliser chamber
- Inhalation valve
- Mouthpiece with valve
e) Connector tube
- Separate the nebuliser top from the bottom by twisting the top counterclockwise and then lifting it. Place the top on the towel and stand the nebuliser bottom upright on the towel.
- Connect one end of the tube to the air outlet of the compressor. Make sure the tube fits perfectly and connect the compressor to the power outlet.
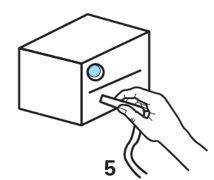
- Open the ampoule by holding the bottom with one hand and twisting the top with the other hand. Be careful not to squeeze the ampoule until you are ready to empty it into the nebuliser chamber.
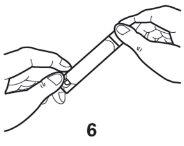
- When you are ready, transfer all the contents of the ampoule into the nebuliser chamber.
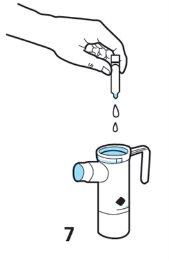
- Replace the nebuliser top. Twist the top clockwise until it fits snugly onto the bottom of the nebuliser.
- Place the mouthpiece at the outlet of the nebuliser. Press the inhalation valve firmly onto the top of the nebuliser. The inhalation valve should fit without effort (consult the PARI LC PLUS nebuliser leaflet).
- Connect the free end of the tube to the air inlet on the bottom of the nebuliser, making sure to keep the nebuliser upright. Press the tube firmly onto the air inlet.
Take your medicine:
- Turn on the compressor
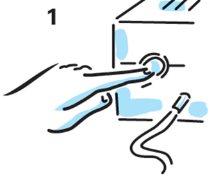
- Check that the mist coming out of the mouthpiece is uniform. If there is no mist, check all tube connections and that the compressor is working correctly.
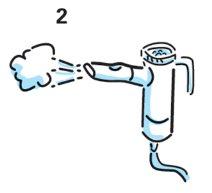
- Sit or stand up to breathe normally.
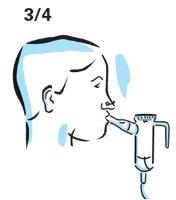
- Place the mouthpiece between your teeth and the top of your tongue. Breathe normally, but only through your mouth (you can use nose clips to help). Try not to block the airflow with your tongue.
- Continue until you have used up all of the Tobramycin Teva Solution for Nebulisation and no more mist is produced.
- Please remember to clean and disinfect the nebuliser after treatment. Never use a dirty or clogged nebuliser. Do not share your nebuliser with others.
It will take 15 minutes to use up the entire contents. You may hear a gurgling sound when the nebuliser chamber is empty. If you are interrupted or need to cough or rest during treatment, turn off the compressor to conserve your medicine. When you are ready to resume treatment, turn the compressor back on.
If you have any further questions about the use of this medicine, consult your doctor or pharmacist.
4. Possible Adverse Effects
Like all medicines, this medicine may cause adverse effects, although not all people suffer from them.
Some adverse effects may be serious:
If you experience any of the following adverse effects, stop using Tobi and inform your doctor immediately:
- Unusual difficulty breathing with wheezing or coughing and chest tightness
- Allergic reactions including hives and itching
If you experience any of the following adverse effects, inform your doctor immediately:
- Hearing loss (ringing in the ears is a potential warning sign of hearing loss), noises (such as whistling) in the ears
Your underlying lung disease may worsen while using Tobi. This may be due to lack of efficacy. Inform your doctor immediately if this occurs.
Some adverse effects are very frequent
These adverse effects may affect more than 1 in 10 people.
- Runny or congested nose, sneezing
- Voice alteration (hoarseness)
- Discoloration of the expectorated substance (sputum)
- Worsening of lung function test results
If any of these affect you severely, inform your doctor.
Some adverse effects are frequent
These adverse effects may affect up to 1 in 10 people.
- Feeling of general discomfort
- Muscle pain
- Voice alteration with sore throat and difficulty swallowing (laryngitis)
If any of these affect you severely, inform your doctor.
Other adverse effects:
- Itching
- Itchy skin rash
- Skin rash
- Loss of voice
- Altered sense of taste
- Sore throat
If any of these affect you severely, inform your doctor.
If you have received Tobi at the same time or after repeated cycles of tobramycin or other injected aminoglycoside antibiotics, hearing loss has been reported as a side effect.
Injecting tobramycin or other aminoglycosides may cause allergic reactions, hearing problems, and kidney problems.
Patients with cystic fibrosis present various symptoms characteristic of the disease. These may even occur while using tobramycin, but they should not appear more frequently or worsen.
Reporting Adverse Effects
If you experience any type of adverse effect, consult your doctor, pharmacist, or nurse, even if it is a possible adverse effect that is not listed in this leaflet. You can also report them directly through the Spanish Pharmacovigilance System for Human Use Medicines: www.notificaram.es. By reporting adverse effects, you can contribute to providing more information on the safety of this medicine.
5. Storage of Tobramycin Teva Nebulizer Solution
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the packaging, bag, and ampoule after CAD. The expiration date is the last day of the month indicated.
Store in a refrigerator (between 2°C and 8°C). Do not freeze. The bags (intact or open) can be stored at room temperature (not above 25°C) for a maximum of 28 days. Store in the original packaging to protect it from light. Never store an open ampoule. Once opened, the ampoule must be used immediately and any remaining product must be discarded.
Do not use this medicine if you observe visible signs of deterioration (turbidity in the solution). Tobramycin may be slightly yellowish and a color variation may be observed; this is not indicative of loss of activity if the solution has been stored as recommended.
Medicines should not be thrown away through wastewater or household waste. Deposit the packaging and medicines you no longer need at the SIGRE Point in the pharmacy. In case of doubt, ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Package Contents and Additional Information
Composition of Tobramycin Teva Nebulizer Solution
- The active ingredient is tobramycin. Each 5 ml ampoule contains 300 mg of tobramycin.
- The other components are: sodium chloride, water for injectable preparations, sulfuric acid (for pH adjustment), and sodium hydroxide (for pH adjustment).
Appearance of the Product and Package Contents
Tobramycin Nebulizer Solution is a clear or slightly yellowish solution.
It is presented in 5 ml single-dose ampoules.
A aluminum bag contains and seals 4 ampoules.
Each package consists of 14 (56 ampoules), 28 (112 ampoules), or 42 (168 ampoules) aluminum bags, sufficient for one, two, or three treatment cycles, respectively.
Only some package sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Holder
Teva Pharma, S.L.U.
C/ Anabel Segura, 11 Edificio Albatros B, 1ª Planta
28108 Alcobendas (Madrid)
Spain
Manufacturer
Norton Healthcare Limited
T/A Ivax Pharmaceuticals UK,
Aston Lane North, Whitehouse Vale Industrial Estate, Preston Brook,
Runcorn, Cheshire, WA7 3FA,
United Kingdom
or
Pharmachemie B.V.
Swensweg 5
2031 GA Haarlem
Netherlands
or
Merckle GmbH
Ludwig-Merckle-Str. 3
89143 Blaubeuren
Germany
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Denmark: Tobramycin TEVA
Germany: Tobramycin Teva® 300 mg/5 ml Steri-Neb® Lösung für einen Vernebler
Ireland: Tobramycin Teva 300 mg/5 ml Nebuliser Solution
Italy: Tobramicina Teva 300 mg/5 ml soluzione per nebulizzatore
Portugal: Tobramicina Teva
Spain: Tobramicina Teva 300 mg/5 ml solución para inhalación por nebulizador
Netherlands: Tobramycine 300 mg/5 ml PCH, vernevelossing
United Kingdom (Northern Ireland): Tymbrineb 300 mg/5 ml Nebuliser Solution
Date of the last revision of this leaflet:February 2022
“Detailed information on this medicine is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.gob.es/”
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to TOBRAMICIN TEVA 300 mg/5 ml SOLUTION FOR NEBULIZER INHALATIONDosage form: PULMONARY INHALATION, 300 mg / 4 mlActive substance: tobramycinManufacturer: Chiesi España S.A.U.Prescription requiredDosage form: PULMONARY INHALATION, 28 mg tobramycinActive substance: tobramycinManufacturer: Viatris Healthcare LimitedPrescription requiredDosage form: PULMONARY INHALATION, 300 mg/5 mlActive substance: tobramycinManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for TOBRAMICIN TEVA 300 mg/5 ml SOLUTION FOR NEBULIZER INHALATION
Discuss questions about TOBRAMICIN TEVA 300 mg/5 ml SOLUTION FOR NEBULIZER INHALATION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















