
Bramitob

Ask a doctor about a prescription for Bramitob

How to use Bramitob
Leaflet accompanying the packaging: patient information
Bramitob, 300 mg/4 ml, solution for nebulisation
Tobramycin
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- If you have any further questions, ask your doctor or pharmacist.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, including any not listed in this leaflet, tell your doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Bramitob and what is it used for
- 2. Important information before using Bramitob
- 3. How to use Bramitob
- 4. Possible side effects
- 5. How to store Bramitob
- 6. Contents of the pack and other information
1. What is Bramitob and what is it used for
Bramitob contains tobramycin, which is an antibiotic belonging to a group of medicines called aminoglycosides. It combats infections caused by bacteria Pseudomonas aeruginosa. Bramitob is used in patients with cystic fibrosis to treat chronic chest infections caused by Pseudomonasbacteria. The medicine works by killing bacteria, which helps to improve breathing. Pseudomonasbacteria are common bacteria that cause infections in almost all patients with cystic fibrosis at some point in their lives. In some patients, the infection occurs later in life, and in others at a very young age. If the infection is not properly controlled, it will cause lung damage and further complications. Since Bramitob is administered by inhalation, the antibiotic - tobramycin - gets directly into the lungs to combat the bacteria that caused the infection. Bramitob is indicated for use only in patients aged 6 years and older. To get the best results from the treatment, you should make every effort to take the medicine as instructed.
2. Important information before using Bramitob
When not to use Bramitob:
- if you are allergic to tobramycin, any of the other ingredients of this medicine (listed in section 6), or to any other aminoglycoside antibiotic;
- if you are taking other medicines listed below in the section "Bramitob and other medicines".
Warnings and precautions
Before starting to use Bramitob, discuss it with your doctor or pharmacist. Tobramycin contained in Bramitob belongs to a group of medicines that may occasionally cause hearing loss, dizziness, and kidney damage (see also section 4 "Possible side effects"). It is important to inform your doctor if you experience any of the following symptoms.
- If you experience chest tightness after using Bramitob. The first dose of Bramitob will be supervised by a doctor who will check your lung function before and after taking the medicine. If you are not already taking a bronchodilator (such as salbutamol), your doctor may recommend using such a medicine before taking Bramitob.
- If you have ever had neurological or muscular disorders, such as parkinsonism or other diseases characterized by muscle weakness, including myasthenia.
- If you have ever had kidney disease. Before starting to use Bramitob, your doctor may check if your kidney function is normal by recommending a blood or urine test. Your doctor may regularly check your kidney function during treatment.
- If you have ever had:
- ringing in the ears,
- any other hearing problems,
- dizziness. Your doctor may check your hearing before starting to use Bramitob and at each stage during treatment.
- If you are currently coughing up blood in your sputum. Inhaled medicines may cause coughing, and your attending doctor may decide to stop using Bramitob until there is only a small amount of blood in your sputum or until the blood in your sputum stops.
- If you are worried that Bramitob is not working as well as it should. Sometimes bacteria can become resistant to the action of the antibiotic.
Bramitob and other medicines
Tell your doctor or pharmacist about all the medicines you are taking, have recently taken, or plan to take, including those obtained without a prescription.
- If you are taking diuretic medicines containing furosemide or ethacrynic acid, you should not use Bramitob without discussing it with your doctor.
- If you are taking urea or taking mannitol intravenously or orally (medicines used in the hospital to treat patients in a severe condition), you should not use Bramitob.
- Some other medicines may occasionally have a harmful effect on the kidneys or hearing, and this effect may be enhanced during the use of Bramitob.
In addition to Bramitob inhalation, you may be given tobramycin or other aminoglycosides by injection. Such injections, which can increase the very low concentration of aminoglycoside in the blood that occurs after taking Bramitob, should be avoided if you are taking the following medicines at the same time:
- amphotericin B, cephalothin, cyclosporin, tacrolimus, polymyxins,
- platinum compounds (e.g. carboplatin or cisplatin),
- cholinesterase inhibitors (e.g. neostigmine or pyridostigmine), botulinum toxin. If this applies to you, you should talk to your doctor.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to have a child, ask your doctor or pharmacist for advice before taking this medicine. It is not known whether inhalation of this medicine by a pregnant woman causes side effects. Tobramycin and other aminoglycoside antibiotics given by injection can harm the unborn baby, for example, by causing deafness and kidney problems. If you are breastfeeding, you should ask your doctor for advice before taking this medicine.
Driving and using machines
Bramitob has a minor influence on the ability to drive and use machines. Bramitob can rarely cause dizziness. For this reason, it is possible that Bramitob will affect the ability to drive and use machines.
3. How to use Bramitob
This medicine should always be used exactly as your doctor has told you. If you are not sure, ask your doctor or pharmacist. The instructions for using Bramitob are given below, after the dosage recommendations. Do notmix or dilute Bramitob with other medicines in the nebulizer. If you are taking several medicines because of cystic fibrosis, you should use them in the following order:
- bronchodilator (e.g. salbutamol), then
- chest physiotherapy, then
- other inhaled medicines, then
- Bramitob. Make sure to ask your doctor about the order of taking the medicines.
Bramitob should be used through a clean, dry PARI LC PLUS or PARI LC SPRINT reusable nebulizer (for personal use) and a suitable compressor. Ask your doctor or physiotherapist which compressor to use. The single-dose container with Bramitob should be opened immediately before use. Any remaining solution, not used immediately, should be discarded.
Dosage
- The dose of the medicine (one 4 ml container) is the same for all patients aged 6 years and older.
- You should use twosingle-dose containers per day for 28 days. You should use the contents of one container in the morning and the second in the evening. You should keep a 12-hour interval between doses.
- Then there is a period of 28 days without using the medicine, and then the next 28-day treatment cycle begins.
- It is important to use the medicine twice a day every day of the 28-day treatment period and to maintain the cycle of 28 days of treatment and 28 days of rest. You should take Bramitob according to this scheme until your doctor decides to stop the medicine.
Using a higher dose of Bramitob than recommended
If you inhale too much Bramitob, you may experience a strong hoarseness. Tell your doctor about it as soon as possible.
Missing a dose of Bramitob
- If it is more than 6 hours until the next dose (container), you should take Bramitob immediately.
- If it is less than 6 hours until the next dose (container), you should skip the missed dose of the medicine. Take the next dose at the usual time.
Stopping the use of Bramitob
If you have any further questions about using this medicine, ask your doctor, pharmacist, or nurse.
Instructions for use
Bramitob is intended for administration via a nebulizer, do not use it in any other way.
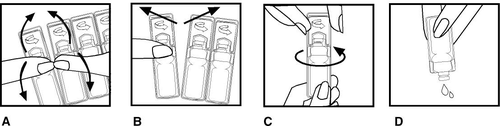
- 1. Before opening the single-dose container according to the instructions below, wash your hands thoroughly with soap and water.
- 2. Bend the single-dose container forward and backward (Fig. A).
- 3. Carefully tear off the new single-dose container from the strip with the remaining containers (first the top, then the middle part of the container), leaving the rest of the containers in the foil pouch (Fig. B).
- 4. Open the single-dose container by twisting the cap in the direction indicated by the arrow (Fig. C).
- 5. Gently squeeze the contents of the container into the nebulizer chamber (Fig. D).
- 6. Turn on the compressor.
- 7. Make sure that a uniform mist is coming out of the mouthpiece.
- 8. Sit or stand upright so that you can breathe normally.
- 9. Place the mouthpiece between your teeth, at the end of your tongue. Breathe normally, but only through your mouth (nasal clips may be helpful). Try not to block the end of the mouthpiece with your tongue.
- 10. Continue these actions until all of Bramitob is used up, which should take about 15 minutes.
- 11. If you need to interrupt the intake of the medicine for a while, or if you need to cough or rest, turn off the compressor so that the medicine is not released. Turn the compressor back on when you are ready to continue taking the medicine.
If you have any questions about using this medicine, ask your doctor or pharmacist.
Maintenance of the nebulizer and compressor
Follow the manufacturer's instructions for the nebulizer and compressor regarding the maintenance and use of the devices.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them. If you are unsure about any of the side effects listed below, ask your doctor for an explanation. The most commonside effects of Bramitob, which may affect more than 1 in 100 people, are: cough, hoarseness. Uncommonside effects of Bramitob, which may affect more than 1 in 1,000 people, include: thrush (yeast infection) in the mouth, dizziness of labyrinthine origin (balance disorders), hearing loss, increased saliva, tongue inflammation, rash, sore throat, and increased liver enzyme activity in the blood, wheezing, nausea, dryness of the mucous membranes, coughing up blood, oral and pharyngeal inflammation, chest pain, hearing loss, headache, shortness of breath, weakness, production of more than normal amounts of sputum (substance coughed up during coughing), stomach pain, and fungal infection. Rareside effects, which may affect more than 1 in 10,000 people, include: loss of appetite, ringing in the ears, stiffness in the chest or difficulty breathing, loss of voice, nosebleeds, runny nose, mouth ulcers, vomiting, taste disorders, asthma, dizziness, weakness, fever and pain, laryngitis (change of voice with pain in the throat and difficulty swallowing). Very rareside effects, which may affect less than 1 in 10,000 people, include: swelling of lymph nodes, drowsiness, ear disorders, ear pain, hyperventilation (accelerated respiratory function of the lungs), sinusitis, diarrhea, allergic reactions, including hives and itching, oxygen deficiency in the blood and tissues of the body (hypoxia), back pain, abdominal pain, and general malaise.
Reporting side effects
If you experience any side effects, including any not listed in this leaflet, tell your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products: Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, Website: https://smz.ezdrowie.gov.pl. Side effects can also be reported to the marketing authorization holder. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Bramitob
- Keep the medicine out of the sight and reach of children.
- For single use only. Do not use this medicine after the expiry date stated on the carton and label after EXP. The expiry date refers to the last day of that month. Bramitob can be used even if the solution changes color. Shelf life during use: foil pouches with Bramitob (unopened or opened) can be stored for up to 3 months at a temperature not exceeding 25 °C.
- Store the medicine in a refrigerator (2-8 °C). If you do not have access to a refrigerator or during transport of the medicine, single-dose containers can be stored for up to 3 months at a temperature not exceeding 25 °C.
- Store the containers in the original packaging to protect them from light.
- After opening the single-dose container: use the medicine immediately.
- After the first use: the single-dose container should be discarded immediately after use.
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the pack and other information
What Bramitob contains
- The active substance of the medicine is tobramycin. Each single-dose container of 4 ml contains 300 mg of tobramycin.
- The other ingredients are: sodium chloride, sulfuric acid, sodium hydroxide (to adjust the pH) and water for injections.
What Bramitob looks like and contents of the pack
The Bramitob solution is yellowish in color. Bramitob, solution for nebulisation, is available in single-dose containers containing 4 ml of solution. Each sealed foil pouch contains 4 containers, and the cardboard boxes contain 16, 28, or 56 single-dose containers. Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder: Chiesi Farmaceutici S.p.A., Via Palermo 26/A, 43122 Parma, Italy. Manufacturer: Chiesi Farmaceutici S.p.A., 96 Via S. Leonardo, 43122 Parma, Italy, Genetic S.p.A., Contrada Canfora, 84084 Fisciano – Salerno, Italy. For further information on this medicine, please contact the local representative of the marketing authorization holder: Chiesi Poland Sp. z o.o., Al. Jerozolimskie 134, 02-305 Warsaw, tel.: (22) 620 14 21, fax: (22) 652 37 79, e-mail: [email protected]
This medicine is authorized in the Member States of the European Economic Area and in the United Kingdom (Northern Ireland) under the following names:
Austria: Bramitob, Czech Republic: Bramitob, Denmark: Bramitob, Finland: Bramitob, Germany: Bramitob, Greece: Bramitob, Hungary: Bramitob, Ireland: Bramitob, Italy: Tobrineb, Netherlands: Bramitob, Poland: Bramitob, Norway: Bramitob, Portugal: Bramitobb, Slovakia: Bramitob, Spain: Bramitob, Sweden: Bramitob, United Kingdom (Northern Ireland): Bramitob, Date of last revision of the leaflet: 10/2023, (logo of the marketing authorization holder)
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterChiesi Farmaceutici S.p.A. Genetic S.p.A
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BramitobDosage form: Solution, 1 mg/mlActive substance: tobramycinManufacturer: B. Braun Medical S.A.Prescription requiredDosage form: Solution, 3 mg/mlActive substance: tobramycinManufacturer: B. Braun Medical S.A.Prescription requiredDosage form: Solution, 300 mg/5 mlActive substance: tobramycinManufacturer: Medichem S.A.Prescription required
Alternatives to Bramitob in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Bramitob in Ukraina
Alternative to Bramitob in Hiszpania
Online doctors for Bramitob
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Bramitob – subject to medical assessment and local rules.














