
VANTOBRA 170 mg SOLUTION FOR NEBULIZER INHALATION


How to use VANTOBRA 170 mg SOLUTION FOR NEBULIZER INHALATION
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet:information for the patient
Vantobra 170 mg solution for inhalation by nebulizer
tobramycin
Read the entire leaflet carefully before starting to use this medicine, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are side effects not listed in this leaflet. See section 4.
Contents of the leaflet
- What is Vantobra and what is it used for
- What you need to know before starting to use Vantobra
- How to use Vantobra
- Possible side effects
- Storage of Vantobra
- Package contents and additional information
1. What is Vantobra and what is it used for
What is Vantobra
Vantobra contains an antibiotic called tobramycin. It belongs to a class of antibiotics known as aminoglycosides.
What is Vantobra used for
Vantobra is used in patients with cystic fibrosis from 6 years of age for the treatment of lung infections caused by a bacterium called Pseudomonas aeruginosa.
Pseudomonas aeruginosais a bacterium that frequently infects the lungs of patients with cystic fibrosis at some point in their lives. If the infection is not treated properly, it will continue to damage the lungs and cause more respiratory problems.
How Vantobra works
When Vantobra is inhaled, the antibiotic can enter the lungs directly to fight the bacteria that cause the infection. This medicine works by altering the production of proteins that bacteria need to build their cell walls. This damages the bacteria and eventually kills them.
2. What you need to know before starting to use Vantobra
Do not use Vantobra:
- if you are allergic (hypersensitive) to tobramycin or to any other aminoglycoside antibiotic or to any of the other components of Vantobra (listed in section 6).
If you are in any of the above circumstances, inform your doctor before using Vantobra.
Warnings and precautions
Consult your doctor if you have ever suffered from any of the following diseases:
- hearing problems (including ringing in the ears and dizziness)
- kidney problems
- chest discomfort
- blood in the sputum (the substance that is expelled when coughing)
- persistent or worsening muscle weakness, a symptom often related to disorders such as myasthenia (muscle weakness) or Parkinson's disease.
If you are in any of the above circumstances, inform your doctor before using Vantobra.
If you have hearing or kidney problems, your doctor may take blood samples to monitor the amount of Vantobra present in your body.
If you or members of your maternal family have a disease caused by a mitochondrial mutation (a genetic disease) or hearing loss due to antibiotics, it is recommended that you inform your doctor or pharmacist before taking this medicine. Certain mitochondrial mutations can increase your risk of hearing loss with this product. Your doctor may recommend genetic tests before administering Vantobra.
Inhaling medications can cause chest discomfort due to the narrowing of the airways; this can happen with Vantobra. Your doctor may ask you to use other medications suitable for widening the airways before using Vantobra.
Pseudomonasstrains can become resistant to antibiotic treatment over time. This means that, over time, Vantobra may stop working as it should. If you have doubts about this, consult your doctor.
If you are also taking tobramycin or another aminoglycoside antibiotic administered by injection, this may increase the risk of experiencing side effects. Your doctor will monitor you as necessary.
Children
This medicine is not indicated for use in children under 6 years of age.
Other medicines and Vantobra
Tell your doctor or pharmacist if you are using or have recently used other medicines, including those purchased without a prescription.
Do not take the following medicines while using Vantobra:
- furosemide, a diuretic (a medicine that increases urine volume)
- other medicines with diuretic potential, such as urea or mannitol
- other medicines that can harm your kidneys or hearing:
- amphotericin B, cephalothin, polymyxins (used to treat microbial infections), cyclosporine, tacrolimus (used to reduce the activity of the immune system). These medicines can harm the kidneys;
- platinum-based compounds, such as carboplatin and cisplatin (used to treat certain types of cancer). These medicines can harm the kidneys or hearing.
The following medicines may increase the risk of harmful effects if administered when you are already receiving tobramycin or another aminoglycoside antibiotic administered by injection:
- anticholinesterases, such as neostigmine and pyridostigmine (used to treat muscle weakness) or botulinum toxin. These medicines can cause or worsen muscle weakness.
If you take any of the above medicines, consult your doctor before using Vantobra.
Do not mix or dilute Vantobra with any other medicine in the Tolero handheld nebulizer provided with Vantobra.
If you are taking different medicines for cystic fibrosis, you will take them in the following order:
- Bronchodilator treatment, such as salbutamol
- Respiratory physiotherapy
- Other inhaled medicines
- Vantobra
Consult your doctor about the order.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor before using this medicine.
It is not known if inhaling this medicine during pregnancy can cause side effects. When administered by injection, tobramycin and other aminoglycoside antibiotics can cause harm to the fetus, such as deafness and kidney problems.
If you are breastfeeding, you should consult your doctor before using this medicine.
Driving and using machines
Vantobra is not expected to affect your ability to drive or use machines.
3. How to use Vantobra
Follow your doctor's instructions for administering this medicine exactly. If in doubt, consult your doctor again.
The recommended dose is two ampoules per day (one in the morning and one in the evening) for 28 days.
- The dose is the same for all people from 6 years of age.
- Inhale the complete contents of one ampoule through the mouth in the morning and another ampoule in the evening using the Tolero handheld nebulizer.
- It is recommended that the interval between doses be as close as possible to 12 hours, but in any case, this interval should be at least 6 hours.
- After using the medicine for 28 days, you will start a 28-day rest period, during which you will not inhale any dose of Vantobra. Then, you will have to start a new cycle after the rest period (as illustrated).
- It is essential that you continue using the medicine twice a day during the 28 days of treatment and respect the cycle of 28 days with treatment and 28 days without treatment.

Repeat the cycle
Continue using Vantobra following this same scheme until your doctor tells you otherwise.
If you have any doubts about how long you should continue using Vantobra, consult your doctor or pharmacist.
Preparing Vantobra for inhalation
- Use Vantobra only with the Tolero handheld nebulizer provided with Vantobra to ensure that you inhale the correct dose. Do not use the Tolero handheld nebulizer with any other medicine.
- Read the instructions for use included with the Tolero handheld nebulizer before using it.
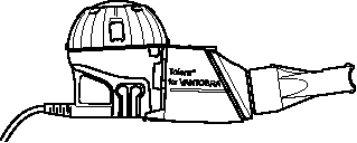
- Make sure you have an eTrack or eBase controller to connect the Tolero handheld nebulizer to. The corresponding controller can be prescribed by your doctor or purchased separately.
- Wash your hands well with soap and water.
- Remove a Vantobra ampoule from the aluminum paper pouch just before inhalation.
- Keep the rest of the medicine refrigerated in the original box.
- Place all the parts of the Tolero handheld nebulizer on a clean and dry paper or cloth. Make sure the nebulizer is on a flat and stable surface.
- Assemble the Tolero handheld nebulizer as indicated in its instructions for use.
- Hold the ampoule upright and gently tap it before removing the top to avoid spilling. Empty the contents of one ampoule into the medication reservoir of the Tolero handheld nebulizer.
- Start the treatment sitting upright in a well-ventilated room. Hold the nebulizer in a horizontal position and breathe through your mouth normally. Avoid breathing through your nose. Continue inhaling and exhaling comfortably until the treatment is finished. When all the medicine has been administered, you will hear an acoustic signal indicating that the administration is complete.
- If for any reason you need to interrupt the treatment before finishing it, hold the On/Off button for one second. To restart the treatment, hold the On/Off button for one second again.
- The Tolero handheld nebulizer must be cleaned and disinfected as described in its instructions for use.
- Use a new Tolero handheld nebulizer for each treatment cycle (28 days of active treatment), including the one provided with the medicine.
Do not use an alternative and untested nebulizer system, as this may alter the amount of medicine that reaches the lungs, which in turn may alter the safety and efficacy of the medicine.
If you use more Vantobra than you should
If you inhale too much Vantobra, your voice may become very hoarse. Inform your doctor as soon as possible. If you swallow Vantobra, it is unlikely to cause serious problems, since tobramycin is hardly absorbed in the stomach, but still, you should inform your doctor as soon as possible.
If you forget to use Vantobra
If you forget to use Vantobra and there are at least 6 hours until the next dose, take the dose as soon as you can. Otherwise, wait until the next dose. Do not use a double dose to make up for forgotten doses.
If you interrupt treatment with Vantobra
Do not interrupt treatment with Vantobra unless your doctor tells you to, as the lung infection may not be sufficiently controlled and could worsen.
If you have any other doubts about using this medicine, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everyone gets them.
Some side effects can be serious
- chest discomfort with difficulty breathing (rare, may affect up to 1 in 1,000 people)
- allergic reactions, such as hives and itching (very rare, may affect up to 1 in 10,000 people)
If you experience any of these effects, stop using Vantobra and contact your doctor immediately.
People with cystic fibrosis have various symptoms of this disease. These symptoms may continue to occur while using Vantobra, but they should not be as frequent or worse than before.
If your underlying lung disease seems to worsen while taking Vantobra, inform your doctor immediately.
Other side effects may be:
Uncommon (may affect up to 1 in 100 people)
- difficulty breathing
- voice alteration (hoarseness)
- increased frequency of coughing
- sore throat
Rare (may affect up to 1 in 1,000 people)
- Laryngitis (inflammation of the vocal cords that can cause voice alteration, sore throat, and difficulty swallowing)
- Loss of voice
- Headache, weakness
- Nasal bleeding, nasal discharge
- Ringing in the ears (usually transient), hearing loss, dizziness
- Coughing up blood, producing more sputum than usual, chest discomfort, asthma, fever
- Taste disturbances, feeling unwell (nausea), mouth ulcers, discomfort (vomiting), decreased appetite
- Rash
- Chest pain or generalized pain
- Worsening of lung function test results
Very rare (may affect up to 1 in 10,000 people)
- Fungal infections in the mouth or throat, such as thrush
- Inflammation of the lymph nodes
- Drowsiness
- Ear pain, ear problems
- Hyperventilation, low oxygen levels in the blood, sinusitis
- Diarrhea, pain in or around the stomach
- Red-colored pustules, papules on the skin
- Hives, itching
- Back pain
- General discomfort
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are possible side effects not listed in this leaflet. You can also report them directly through the national reporting system included in Appendix V. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Vantobra
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiration date that appears on the ampoule, on the pouch, or on the box after "EXP". The expiration date is the last day of the month indicated.
Store in a refrigerator (between 2°C and 8°C). If you do not have a refrigerator (for example, when transporting the medicine), you can store the box with the medicine (even if the pouches are open) below 25°C for a maximum of 4 weeks. If the medicine is stored at room temperature for more than 4 weeks, it will be discarded according to local regulations.
Do not use this medicine if it appears cloudy or particles are observed in the solution.
Never store an open ampoule.Once an ampoule is opened, it must be used immediately, and the remaining product will be discarded.
Medicines should not be thrown away in the trash. Ask your pharmacist how to dispose of the packaging and medicines you no longer need. This will help protect the environment.
6. Package contents and additional information
Composition of Vantobra
- The active ingredient is tobramycin. One ampoule contains 170 mg of tobramycin as a single dose.
- The other components (excipients) are: sodium chloride, calcium chloride, magnesium sulfate, water for injectable preparations, sulfuric acid, and sodium hydroxide to adjust the pH.
Appearance of Vantobra and package contents
Vantobra solution for inhalation by nebulizer is supplied in a ready-to-use ampoule.
Vantobra is a clear to yellowish solution, which may vary to dark yellow. This does not affect the action of Vantobra as long as the storage instructions have been followed.
The ampoules are packaged in pouches, and each pouch contains 8 ampoules, which corresponds to four days of treatment.
Vantobra is available together with a Tolero handheld nebulizer. It is presented in a box that contains two boxes inside, one with the medicine (56 ampoules with solution for inhalation by nebulizer in 7 pouches) and another that contains the Tolero handheld nebulizer. One package is sufficient for a 28-day treatment cycle.
Marketing authorization holder and manufacturer
PARI Pharma GmbH
Moosstrasse 3
D-82319 Starnberg
Germany
Tel.: +49 (0) 89 – 74 28 46 - 10
Fax: +49 (0) 89 – 74 28 46 30
E-mail: [email protected]
Date of the last revision of this leaflet:
Other sources of information
Detailed information about this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu. There are also links to other websites about rare diseases and orphan medicines.
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to VANTOBRA 170 mg SOLUTION FOR NEBULIZER INHALATIONDosage form: PULMONARY INHALATION, 300 mg / 4 mlActive substance: tobramycinManufacturer: Chiesi España S.A.U.Prescription requiredDosage form: PULMONARY INHALATION, 28 mg tobramycinActive substance: tobramycinManufacturer: Viatris Healthcare LimitedPrescription requiredDosage form: PULMONARY INHALATION, 300 mg/5 mlActive substance: tobramycinManufacturer: Accord Healthcare S.L.U.Prescription required
Online doctors for VANTOBRA 170 mg SOLUTION FOR NEBULIZER INHALATION
Discuss questions about VANTOBRA 170 mg SOLUTION FOR NEBULIZER INHALATION, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















