
Tobramicin Via pharma

Ask a doctor about a prescription for Tobramicin Via pharma

How to use Tobramicin Via pharma
Leaflet accompanying the packaging: patient information
Tobramycin Via pharma, 300 mg/5 ml solution for nebulisation
Tobramycin
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, so you can read it again if you need to.
- If you have any doubts, consult your doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same as yours.
- If the patient experiences any side effects, including those not listed in this leaflet, they should tell their doctor or pharmacist. See section 4.
Table of contents of the leaflet
- 1. What is Tobramycin Via pharma and what is it used for
- 2. Important information before using Tobramycin Via pharma
- 3. How to use Tobramycin Via pharma
- 4. Possible side effects
- 5. How to store Tobramycin Via pharma
- 6. Contents of the packaging and other information
1. What is Tobramycin Via pharma and what is it used for
Tobramycin Via pharma is an aminoglycoside antibiotic.
Tobramycin Via pharma is used in patients with cystic fibrosis aged 6 years and older
to treat lung infections caused by the bacterium called Pseudomonas aeruginosa.
Tobramycin Via pharma combats the lung infection caused by Pseudomonasbacteria and makes it easier
for the patient to breathe.
When inhaling Tobramycin Via pharma, the antibiotic goes directly to the lungs to
combat the bacteria causing the infection. To get the best results from this
medicine, it should be used according to the instructions in the leaflet.
What is Pseudomonas aeruginosa?
It is a very common bacterium that almost every cystic fibrosis patient will get a lung infection from at some point in their life. Some patients get infected later in life, while others get infected at a very young age.
This is one of the most harmful bacteria for cystic fibrosis patients. If the infection is not
treated properly, it will lead to progressive lung damage, causing further breathing problems.
Tobramycin Via pharma kills Pseudomonasbacteria that cause lung infections. The infection can be effectively treated, especially if it is detected early.
2. Important information before using Tobramycin Via pharma
When not to use Tobramycin Via pharma:
- if the patient is allergic to tobramycin or to any other aminoglycoside antibiotic, or to any of the other ingredients of this medicine (listed in section 6).
If the patient has any of the following conditions, they should tell their doctor before using Tobramycin Via pharma.
Warnings and precautions
Before starting to use Tobramycin Via pharma, the patient should discuss with their doctor or pharmacist if they have ever had any of the following conditions:
- Hearing problems (including ringing in the ears and dizziness)
- Kidney disease
- Unusual breathing difficulties with wheezing or coughing and chest tightness
- Blood in sputum (coughed-up phlegm)
- Muscle weakness that persists or worsens over time; this is a symptom most commonly associated with diseases such as myasthenia or Parkinson's disease.
Inhaled medicines can cause chest tightness and wheezing; these symptoms may occur when using Tobramycin Via pharma. The first dose of Tobramycin Via pharma should be taken under medical supervision and the doctor will assess lung function before and after administration. If the patient has not previously used a bronchodilator (such as salbutamol), the doctor may recommend taking such a medicine before taking Tobramycin Via pharma.
If the patient is taking Tobramycin Via pharma, Pseudomonas aeruginosastrains may become resistant to treatment over time. This may mean that the medicine may not work as well as it should. If concerned, the patient should talk to their doctor.
Tobramycin given by injection can sometimes cause hearing loss, dizziness, and kidney damage, and may also harm the unborn baby.
Children and adolescents
Tobramycin Via pharma can be used in children and adolescents aged 6 years and older.
Tobramycin Via pharma should not be given to children under 6 years of age.
Tobramycin Via pharma and other medicines
The patient should tell their doctor or pharmacist about all medicines they are taking, have recently taken, or might take.
The following medicines should not be taken while using Tobramycin Via pharma:
- Furosemide (a diuretic).
- Urea or mannitol.
- Other medicines that can harm the nervous system, kidneys, or hearing.
The following medicines may increase the risk of side effects if taken while the patient is receiving injectionsof tobramycin:
- Amphotericin B, cephalothin, cyclosporin, tacrolimus, polymyxins: these medicines can harm the kidneys.
- Platinum compounds (such as carboplatin and cisplatin) these medicines can harm the kidneys or hearing.
- Cholinesterase inhibitors (such as neostigmine and pyridostigmine) or botulinum toxin: these medicines can cause or worsen muscle weakness. If the patient is taking any of these medicines, they should talk to their doctor before starting Tobramycin Via pharma.
Tobramycin Via pharma should not be mixed or diluted with any other medicines in a nebuliser.
If the patient is taking several different medicines for cystic fibrosis, they should follow the order below:
- 1. a bronchodilator, such as salbutamol
- 2. chest physiotherapy
- 3. other inhaled medicines
- 4. then Tobramycin Via pharma The order should be checked with the doctor.
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a baby, they should ask their doctor for advice before taking this medicine.
It is not known whether using Tobramycin Via pharma by inhalation in pregnant women may cause side effects.
Tobramycin and other aminoglycoside antibiotics given by injection can harm the unborn baby, for example by causing deafness.
Driving and using machines
Tobramycin Via pharma is unlikely to affect the ability to drive or use machines.
3. How to use Tobramycin Via pharma
This medicine should always be used as directed by the doctor. If unsure, the patient should ask their doctor.
What dose and how often to take Tobramycin Via pharma
- The recommended dose is the same for all patients aged 6 years and older
- Use twoampoules per day for 28 days. Inhale the contents of one ampoule in the morning and one in the evening. It is best to keep a 12-hour gap between doses.
- The gap between two consecutive doses of Tobramycin Via pharma must be at least 6 hours.
- After 28 days of taking the medicine, there is a 28-day break during which Tobramycin Via pharma is not taken. After the break, the next treatment cycle begins.
- It is important to take the medicine regularly, twice a day, during the 28-day treatment period and to follow the cycle with a 28-day treatment period and a 28-day breakfrom taking the medicine.
Taking Tobramycin Via pharma
Break in taking Tobramycin Via pharma
Tobramycin Via pharma
Take Tobramycin Via pharma every day, twice a day
Via pharma
Do not take Tobramycin Via pharma for the next 28 days, for 28 days
Repeat the cycle
Using a higher dose of Tobramycin Via pharma than recommended
If the patient inhales too much Tobramycin Via pharma, they may get a hoarse voice.
The patient should tell their doctor as soon as possible. If the patient swallows Tobramycin Via pharma, they should tell their doctor as soon as possible.
Missing a dose of Tobramycin Via pharma
If the patient misses a dose of Tobramycin Via pharma and it is at least 6 hours before the next dose is due, they should take the missed dose as soon as possible.
Otherwise, the patient should wait until the next dose is due. The patient should not take a double dose to make up for the missed dose.
Devices needed to inhale Tobramycin Via pharma
Tobramycin Via pharma should be used with a clean and dry reusable nebuliser.
The LC PLUS nebuliser (manufactured by PARI GmbH) is suitable for use with Tobramycin Via pharma.
The doctor or physiotherapist can instruct the patient on how to use Tobramycin Via pharma correctly and what device is needed. For other medicines used in cystic fibrosis, the patient may need different types of nebulisers.
Preparing to inhale Tobramycin Via pharma
- Wash hands thoroughly with soap and water.
- Each foil sachet contains 7 ampoules of Tobramycin Via pharma. To open the sachet, tear or cut it. Remove one ampoule of Tobramycin Via pharma from the sachet. Put the remaining ampoules back in the sachet and store them in the refrigerator.
- Lay out all the parts of the nebuliser on a clean, dry paper towel or cloth.
- Check that the correct compressor and tubes are available to connect the nebuliser to the compressor.
- Follow the instructions for use provided with the nebuliser. Read the leaflet provided by the manufacturer. Before starting to take the medicine, check that the nebuliser and compressor are working properly, as instructed by the manufacturer.
Using Tobramycin Via pharma with the LC PLUS nebuliser (PARI GmbH)
More detailed information on using the nebuliser can be found in the leaflet provided with the PARI LC PLUS device.
1.
Disconnect the top and bottom parts of the nebuliser by twisting the top part in the opposite direction to the arrow and then lifting it. Place the top part on a towel and stand the bottom part on the towel vertically.
2.
Connect one end of the tube to the outlet of the compressor. Make sure the tube fits tightly. Connect the compressor to the power socket.
3.
Open the ampoule of Tobramycin Via pharma by holding the bottom part with one hand and twisting and breaking off the top with the other hand. Squeeze the entire contents of the ampoule into the bottom part of the nebuliser.
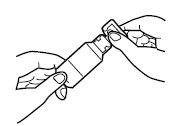
4.
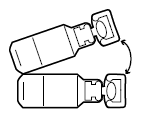
Screw the top part of the nebuliser back onto the bottom part. Put the mouthpiece and vent inhaler in place on the nebuliser. Then connect the compressor according to the instructions for use provided with the PARI LC PLUS nebuliser.
5.
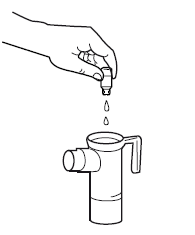
Turn on the compressor. Make sure that a uniform mist is coming out of the mouthpiece. If there is no mist, check all connections and make sure the compressor is working properly.
6.
Sit or stand up straight so that you can breathe properly.
7.
Put the mouthpiece between your teeth, on your tongue. Breathe normally, but only through your mouth (you can use nose clips if your doctor says you can). Try not to block the airflow with your tongue.
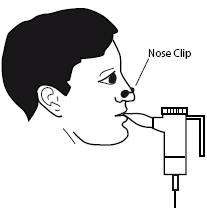
8.
Continue until all of the Tobramycin Via pharma has been used up and the mist stops. Taking the whole dose should take about 15 minutes. After the nebuliser is empty, a clicking sound may be heard.
9. Remember to clean and disinfect the nebuliser after taking the medicine, as instructed by the manufacturer. Never use a nebuliser that is dirty or clogged. The nebuliser is for personal use and should not be used by other people.
If the treatment is interrupted, or if the patient needs to cough or rest during the treatment, they should turn off the compressor to save the medicine for later.
The compressor should be turned back on when the patient can resume taking the medicine. If there are less than 6 hours before the next dose is due, the patient should miss the remaining dose.
4. Possible side effects
Like all medicines, Tobramycin Via pharma can cause side effects, although not everybody gets them.
Some side effects may be serious.
If the patient experiences any of the following symptoms, they should stop using Tobramycin Via pharma and tell their doctor immediately:
- unusual breathing difficulties with wheezing or coughing and chest tightness
- allergic reactions, including hives and itching (very rare).
If the patient experiences any of the following symptoms, they should tell their doctor immediately:
- hearing loss (ringing in the ears is a possible symptom of hearing loss), noises (such as ringing) in the ears
The patient's underlying lung disease may worsen while using Tobramycin Via pharma. This may be due to lack of effectiveness of the treatment. If this happens, the patient should tell their doctor immediately.
Some side effects are very common
These may affect more than 1 in 10 people
- nasal discharge or feeling of nasal congestion, sneezing
- voice changes (hoarseness)
- change in the colour of sputum (phlegm)
- worsening of lung function test results If any of these symptoms are severe, the patient should tell their doctor.
Some side effects are common
These may affect less than 1 in 10 people
- general feeling of being unwell
- muscle pain
- voice changes with sore throat and difficulty swallowing (laryngitis) If any of these symptoms are severe, the patient should tell their doctor.
Other side effects:
- itching
- itchy rash
- rash
- loss of voice
- taste disturbances
- sore throat If any of these symptoms are severe, the patient should tell their doctor.
Hearing loss as a side effect has been reported in cases where Tobramycin Via pharma was used at the same time as or after multiple cycles of treatment with tobramycin or other aminoglycoside antibiotics given by injection.
Administration of tobramycin or other aminoglycosides by injection can cause allergic reactions, hearing problems, and kidney problems.
In patients with cystic fibrosis, many symptoms of the disease may occur. These symptoms may continue to occur while using Tobramycin Via pharma, but they should not occur more frequently or be more severe than before.
Reporting side effects
If the patient experiences any side effects, including those not listed in this leaflet, they should tell their doctor or pharmacist. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
Phone: +48 22 49 29 301
Fax: +48 22 49 29 309
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorisation holder.
By reporting side effects, more information can be gathered on the safety of the medicine.
5. How to store Tobramycin Via pharma
Keep the medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the carton, outer packaging, or ampoule. The expiry date refers to the last day of the month.
Do not use Tobramycin Via pharma if the solution is cloudy or contains particles.
Store in a refrigerator (2°C - 8°C). If there is no access to a refrigerator (for example, when transporting the medicine), the product in the foil sachet (unopened or opened) can be stored at room temperature (not above 25°C) for up to 28 days.
Do not use Tobramycin Via pharma if the ampoules have been stored at room temperature for more than 28 days.
Tobramycin Via pharma is usually a light yellow colour, but its colour may vary and may sometimes be darker. This does not affect the quality of Tobramycin Via pharma if it has been stored as recommended.
Never store an opened ampoule. After opening the ampoule, it should be used immediately, and any remaining product should be discarded.
Medicines should not be disposed of via wastewater or household waste. The patient should ask their pharmacist how to dispose of medicines that are no longer needed. This will help protect the environment.
6. Contents of the packaging and other information
What Tobramycin Via pharma contains
- The active substance is tobramycin. One 5 ml ampoule contains 300 mg of tobramycin, which is a single dose of the medicine.
- The other ingredients are: sodium chloride, water for injections, sodium hydroxide 1N, and sulfuric acid 2N (to adjust the acidity of the solution).
What Tobramycin Via pharma looks like and contents of the pack
Tobramycin Via pharma is a clear, light yellow solution in a ready-to-use ampoule.
The ampoules are packed in foil sachets. One foil sachet contains 7 ampoules.
Tobramycin Via pharma is available in packs containing 56 ampoules. This quantity is sufficient for one treatment cycle.
Marketing authorisation holder and manufacturer
The marketing authorisation holder is:
UAB Via pharma
J.Galvydžio g. 5
LT-08236 Vilnius
Lithuania
The manufacturer is:
MEDICHEM S.A
Narcís Monturiol, 41 A
Sant Joan Despí, 08970 Barcelona
Spain
The medicinal product is available in the Member States of the European Economic Area under the following names:
| Bulgaria | Тобрамицин Виа фарма 300 mg/ 5 ml |
| Estonia | Tobramycin Via pharma |
| Lithuania | Tobramycin Via pharma 300 mg/ 5 ml purškiamasis įkvepiamasis tirpalas |
| Latvia | Tobramycin Via pharma 300 mg/ 5 ml sķīdums izsmidzināšanai |
| Poland | Tobramycin Via pharma |
| Romania | Tobramicină Via pharma 300 mg/ 5 ml soluţie pentru inhalare prin nebulizator |
| Hungary | Tobramycin Via pharma 300 mg/ 5 ml oldat porlasztásra |
Date of last revision of the leaflet:
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterMedichem S.A.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to Tobramicin Via pharmaDosage form: Solution, 300 mg/4 mlActive substance: tobramycinManufacturer: Chiesi Farmaceutici S.p.A. Genetic S.p.APrescription requiredDosage form: Solution, 1 mg/mlActive substance: tobramycinManufacturer: B. Braun Medical S.A.Prescription requiredDosage form: Solution, 3 mg/mlActive substance: tobramycinManufacturer: B. Braun Medical S.A.Prescription required
Alternatives to Tobramicin Via pharma in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Tobramicin Via pharma in Ukraina
Alternative to Tobramicin Via pharma in Hiszpania
Online doctors for Tobramicin Via pharma
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Tobramicin Via pharma – subject to medical assessment and local rules.














