
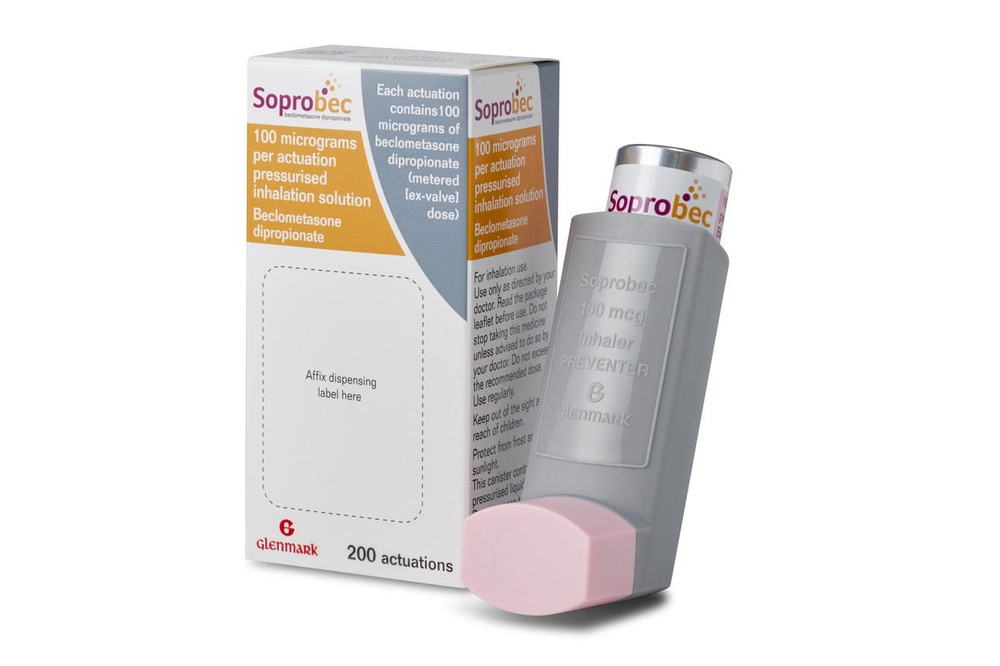
Soprobec

Ask a doctor about a prescription for Soprobec

How to use Soprobec
Leaflet accompanying the packaging: information for the user
Soprobec, 50 micrograms/dose, inhalation aerosol, solution
Soprobec, 100 micrograms/dose, inhalation aerosol, solution
Soprobec, 200 micrograms/dose, inhalation aerosol, solution
Soprobec, 250 micrograms/dose, inhalation aerosol, solution
Beclometasone dipropionate
Read the leaflet carefully before using the medicine, as it contains important information for the patient.
- Keep this leaflet, you may need to read it again.
- In case of any doubts, consult a doctor, pharmacist or nurse.
- This medicine has been prescribed specifically for this person. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any side effects not listed in this leaflet, they should tell their doctor, pharmacist or nurse. See section 4.
Table of contents of the leaflet
- 1. What is Soprobec and what is it used for
- 2. Important information before using Soprobec
- 3. How to use Soprobec
- 4. Possible side effects
- 5. How to store Soprobec
- 6. Package contents and other information
1. What is Soprobec and what is it used for
Soprobec inhalation aerosol, solution is used for the preventionof asthma symptoms. The active substance, beclometasone dipropionate, belongs to a group of medicines called corticosteroids, which are often referred to as steroids. Steroids have anti-inflammatory effects, reducing swelling and irritation in the walls of the small airways in the lungs and making it easier to breathe.
2. Important information before using Soprobec
When not to use Soprobec:
- for the treatment of sudden asthma attacks. The medicine will not help. In this situation, a fast-acting inhalation medicine (rescue medicine) should be used, which should be carried with you at all times.
Warnings and precautions
Before starting treatment with Soprobec, discuss with your doctor, pharmacist or nurse:
You should also discuss with your doctor, pharmacist or nurse if the following situations apply to the patient:
- after switching from steroid tablets to an inhaled form, even if the patient feels an improvement in their airways, they may feel unwell overall, may experience a rash, eczema, nasal discharge (runny nose) and sneezing (rhinitis). Do not stopusing the inhaled medicine unless the doctor advises you to.
If the patient is using high doses of inhaled steroids for a long time and finds themselves in a stressful situation, they may need to be given a steroid medicine in the form of tablets or injections. For example, when admitted to hospital after a serious accident, before surgery, during an acute asthma attack, or if the patient has a respiratory infection, or after another serious illness. The doctor will decide whether additional steroid treatment is necessary and advise how long to take the steroid tablets and how to reduce the dose if the patient feels better.
If the patient experiences blurred vision or other vision disturbances, they should contact their doctor.
Soprobec and other medicines
Before starting treatment, tell your doctor about all medicines you are currently taking or have recently taken, as well as any medicines you plan to take, including those available without a prescription.
Tell your doctor if you are taking disulfiram or metronidazole, due to the possible risk of interaction, especially in sensitive individuals.
Remember to take this medicine and the inhaler with you if you need to go to the hospital.
Some medicines may enhance the effect of Soprobec, and your doctor may advise careful monitoring of your health if you are taking such medicines (including some HIV medicines: ritonavir, cobicistat).
Pregnancy and breastfeeding
If the patient is pregnant or breastfeeding, thinks they may be pregnant or plans to have a child, they should consult their doctor or pharmacist before using this medicine.
Driving and using machines
It is unlikely that Soprobec will affect your ability to drive or use machines.
Soprobec contains alcohol
This medicine contains 7.47 mg (50 micrograms and 100 micrograms strength), 8.05 mg (200 micrograms strength) and 8.62 mg (250 micrograms strength) of alcohol (ethanol) per dose, which is equivalent to 13% w/w (50 micrograms and 100 micrograms strength), 14% w/w (200 micrograms strength) and 15% w/w (250 micrograms strength). The amount of alcohol in each dose of this medicine is equivalent to less than 4 ml of beer or 2 ml of wine. The small amount of alcohol in this medicine will not have noticeable effects.
3. How to use Soprobec
Soprobec is available in 4 different strengths. The doctor will determine the appropriate strength of the medicine for the patient. The 200 micrograms and 250 micrograms strengths are not suitable for children.
The inhaler should always be used as directed by the doctor or pharmacist. If you have any doubts, consult your doctor or pharmacist. The instructions for using the inhaler are in the section on dosage. It may take a few days for the medicine to start working, so it is essential to use it regularly.
Do not stoptreatment, even if you feel better, unless your doctor advises you to. Do notsuddenly stopusing the inhaler.
While using Soprobec, your doctor may regularly assess your asthma symptoms by performing a simple test to assess lung function and ordering blood tests from time to time.
Dosage:
The initial dose will be determined by the doctor, depending on the severity of the asthma symptoms. It may be higher than the dose given below. The doctor will prescribe the smallest dose of Soprobec that will control the symptoms.
A device known as a Volumatic inhalation chamber should always be used:
- when Soprobec is used in adults, the elderly and adolescents aged 16 and over, and the total daily dose is 1000 micrograms or more,
- when Soprobec is used in children and adolescents under 16, who have been prescribed any dose.
Soprobec 50 micrograms
The usual initial dose is:
Adults and the elderly:
200 micrograms (4 puffs) twice a day
Children:
100 micrograms (2 puffs) twice a day
The usual maximum daily dose is:
Adults and the elderly:
800 micrograms (16 puffs)
Children:
400 micrograms (8 puffs)
The total daily dose can be divided into 2, 3 or 4 doses per day.
Soprobec 100 micrograms
The usual initial dose is:
Adults and the elderly:
200 micrograms (2 puffs) twice a day
Children:
100 micrograms (1 puff) twice a day
The usual maximum daily dose is:
Adults and the elderly:
800 micrograms (8 puffs)
Children:
400 micrograms (4 puffs)
The total daily dose can be divided into 2, 3 or 4 doses per day.
Soprobec 200 micrograms
The usual initial dose is:
Adults and the elderly:
200 micrograms (1 puff) twice a day
The usual maximum daily dose is:
800 micrograms (4 puffs)
The total daily dose can be divided into 2, 3 or 4 doses per day.
This strength is not suitable for children.
Soprobec 250 micrograms
The usual initial dose is:
Adults and the elderly:
500 micrograms (2 puffs) twice a day
The usual maximum daily dose is:
2000 micrograms (8 puffs)
The total daily dose can be divided into 2, 3 or 4 doses per day.
This strength is not suitable for children.
Using a higher dose of Soprobec than recommended
Tell your doctor as soon as possible. The doctor may advise a blood test to measure the level of cortisol in the patient's blood and therefore may advise a blood test (cortisol is a steroid hormone that occurs naturally in the body).
It is essential to use the dose as advised by the doctor. Do not increase or decrease the dose of the medicine without consulting your doctor.
Missing a dose of Soprobec
Do not take a double dose to make up for a missed dose. Take the next dose at the right time. Do not take more puffs than prescribed.
Method of administration
Soprobec is for inhalation use.
Instructions for use
It is essential that the patient knows how to use the inhaler correctly. The doctor, nurse or pharmacist will instruct the patient on how to use the inhaler and will regularly check that the patient is using it correctly. Follow the instructions carefully so that the patient knows how when and how manypuffs of the medicine to use and how long to use the inhaler. If you have any doubts about using the inhaler or inhalation, consult your doctor, nurse or pharmacist for help.
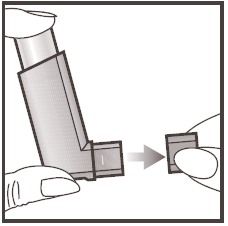
- 1. Remove the protective cap from the mouthpiece by holding it with your thumb and index finger, gently squeeze and pull it off as shown in the picture. Check that the mouthpiece is clean and free of debris, both inside and outside.
Checking the inhaler:If the inhaler is new or has not been used for at least 3 days, you should release one puff away from you (into the air) to check that it is working.
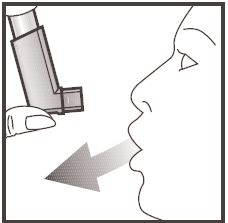
- 2. Hold the inhaler upright as shown in the picture, holding the base of the inhaler with your thumb under the mouthpiece. Take a deep breath out without feeling uncomfortable.
- 3. Place the mouthpiece in your mouth between your teeth and enclose it with your lips, but do not bite it.
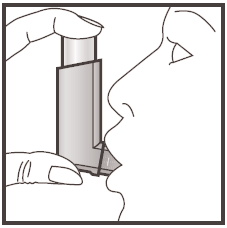
- 4. As you start to breathe in through your mouth, press the top of the inhaler to release one puff, continuing to breathe in slowly and deeply.
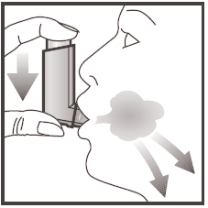
- 5. Hold your breath; remove the inhaler from your mouth and take your finger off the top of the inhaler. Continue to hold your breath for a few seconds or as long as is comfortable. Breathe out slowly.
- 6. If a second puff is needed, hold the inhaler upright for about half a minute, then repeat steps 2 to 5.
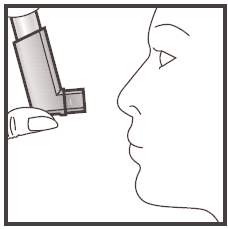
- 7. After use, always replace the protective cap to protect the mouthpiece from dust and dirt. The cap should be pushed on firmly until it clicks into place.
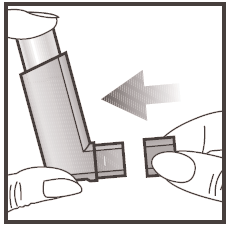
Important: The steps described in points 2, 3, 4 and 5 should not be performed too quickly.
It is essential to start breathing in as slowly as possible, just before activating the inhaler. Before using the inhaler for the first time, practice these steps in front of a mirror.
If the patient notices a "mist" coming out of the top of the inhaler or the sides of the mouth,
this means that the medicine is not getting into the lungs as it should. Take another puff, following the instructions from step 2.
If patients have weak hands or the inhaler is being used by children, it may be easier for them to hold the inhaler with both hands. Place your index fingers on the top of the inhaler and your thumbs on the bottom of the inhaler under the mouthpiece.
If the patient finds it difficult to use the inhaler while breathing in, they may use a Volumatic inhalation chamber. Consult your doctor, pharmacist or nurse about this device.
However, the Volumatic inhalation chamber should always be used if:
- Soprobec is used in adults, the elderly and adolescents aged 16 and over, and the total daily dose is 1000 micrograms or more,
- Soprobec is used in children and adolescents under 16, who have been prescribed any dose.
Young children may find it difficult to use the inhaler correctly and may need help. In children under 5, using the inhaler with a Volumatic inhalation chamber and a face mask may be helpful. Consult your doctor, nurse or pharmacist if the patient has any difficulties.
Cleaning the inhaler
It is essential to clean the inhaler at least once a week to prevent it from becoming blocked.
- Remove the metal canister from the plastic inhaler and remove the protective cap from the mouthpiece.
- Wash the plastic inhaler and the mouthpiece protective cap in warm water. If you use a mild detergent for washing, rinse it thoroughly before drying. Do not put the metal canister in water.
- Leave to dry in a warm place. Avoid excessive heat.
- Put the canister back and replace the mouthpiece protective cap.
It is essential to read the leaflet accompanying the Volumatic inhalation chamber and follow the instructions for its use and cleaning carefully.
4. Possible side effects
Like all medicines, Soprobec can cause side effects, although not everybody gets them.
Stop using Soprobec and contact your doctor immediately if:
- the patient experiences allergic reactions, including skin rash, urticaria, itching or redness of the skin, or swelling of the face, eyes, lips and throat.
- after using the medicine, the patient's wheezing, shortness of breath and cough suddenly worsen.Stopusing Soprobec and use a fast-acting inhalation medicine (rescue medicine) immediately. Contact your doctor immediately. The doctor will assess the patient's asthma symptoms and may decide to change the treatment or prescribe another inhalation medicine for asthma.
The following side effects have been reported. If the patient experiences any side effects, tell your doctor as soon as possible, but do not stoptreatmentunless your doctor advises you to. To prevent side effects, the doctor will prescribe the smallest possible dose of Soprobec to control the asthma symptoms.
Very common (may affect more than 1 in 10 people)
- Fungal infection (candidiasis) in the mouth and (or) throat (thrush). The occurrence of this side effect is more likely if the daily dose is greater than 400 micrograms. Thrush can be treated with antifungal medicines while the patient continues to use Soprobec. To prevent fungal infections in the mouth and throat, brush your teeth with a toothbrush or rinse your mouth with water after each dose of the medicine.
Common (may affect up to 1 in 10 people)
- Hoarseness or sore throat or tongue. To prevent these symptoms, use a Volumatic inhalation chamber or rinse your mouth with water after each use of the inhaler.
Uncommon (may affect up to 1 in 100 people)
- Rash, urticaria, itching and (or) redness of the skin.
Rare (may affect up to 1 in 10,000 people)
- Allergic reactions, including swelling of the eyelids, face, lips and (or) throat (angioedema).
- Breathing difficulties, such as shortness of breath (feeling of shallow breathing or difficulty breathing) and (or) bronchospasm (constriction of the airway walls with reduced airflow).
- Anaphylactic and anaphylactoid reactions (severe allergic reactions that can cause breathing difficulties or affect consciousness).
- Children and adolescents may grow more slowly, so the doctor may regularly check their growth rate. This may happen if Soprobec is used in high doses for a long time.
- Rounded face (moon face) (Cushing's syndrome).
- Decreased bone density (thinning and weakening of bones).
- Eyelid problems, including cataracts and glaucoma (increased pressure in the eyeball).
- Paradoxical bronchospasm.
Frequency not known (frequency cannot be estimated from the available data)
- Sleep problems, depression or feelings of anxiety, restlessness, agitation, excitement or irritability. The occurrence of these symptoms is more likely in children.
Blurred vision.
Headache.
Nausea.
If the patient feels worseor experiences symptoms such as loss of appetite, abdominal pain, weight loss, fatigue, nausea (vomiting), feels weak, sweats, and trembles, they should contact their doctor. This is especially important if the patient is in a stressful situation, such as surgery, infection, acute asthma attack, or another serious illness.
The doctor may advise blood tests from time to time to measure the level of steroids in the patient's body.
Reporting side effects
If you experience any side effects, including any side effects not listed in the leaflet, tell your doctor, pharmacist or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices and Biocidal Products:
Al. Jerozolimskie 181C, 02-222 Warsaw, tel.: +48 22 49 21 301, fax: +48 22 49 21 309,
Website: https://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Soprobec
Keep the medicine out of the sight and reach of children.
- Do not use Soprobec after the expiry date stated on the carton after EXP or on the inhaler label after the abbreviation: "EXP". The expiry date refers to the last day of the month stated.
- Do not freeze.
- Store in the original packaging to protect from light.
- If the inhaler is very cold, remove the metal canister from the plastic inhaler and warm it in your hands for a few minutes neverwarm it in any other way.
- Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
Warning:The canister contains a pressurized liquid. Store away from heat sources and protect from direct sunlight, do not expose to high temperatures (above 50°C) and do not pierce or burn, even after use.
6. Package contents and other information
What Soprobec contains
The active substance of the inhaled medicine is beclometasone dipropionate. Each delivered dose contains 50, 100, 200 or 250 micrograms of beclometasone dipropionate.
The other ingredients are: glycerol, anhydrous ethanol and norfluran (HFC-134a).
This medicine contains fluorinated greenhouse gases.
Soprobec, 50 micrograms:
Each inhaler contains 11.808 g HFC-134a, which is equivalent to 0.017 tons of CO2 equivalent (GWP = 1430)
Soprobec, 100 micrograms:
Each inhaler contains 11.796 g HFC-134a, which is equivalent to 0.017 tons of CO2 equivalent (GWP = 1430)
Soprobec, 150 micrograms:
Each inhaler contains 11.634 g HFC-134a, which is equivalent to 0.017 tons of CO2 equivalent (GWP = 1430)
Soprobec, 200 micrograms:
Each inhaler contains 11.484 g HFC-134a, which is equivalent to 0.016 tons of CO2 equivalent (GWP = 1430)
What Soprobec looks like and contents of the pack
Soprobec 50 micrograms/dose is an inhalation aerosol solution in an aluminum pressurized canister with a cream-colored plastic inhaler and a dark brown protective cap. Each carton contains one inhaler or two inhalers. Each canister contains 200 doses (puffs).
Soprobec 100 micrograms/dose is an inhalation aerosol solution in an aluminum pressurized canister with a gray plastic inhaler and a light pink protective cap. Each carton contains one inhaler or two inhalers. Each canister contains 200 doses (puffs).
Soprobec 200 micrograms/dose is an inhalation aerosol solution in an aluminum pressurized canister with a pink plastic inhaler and a red protective cap. Each carton contains one inhaler or two inhalers. Each canister contains 200 doses (puffs).
Soprobec 250 micrograms/dose is an inhalation aerosol solution in an aluminum pressurized canister with a burgundy-colored plastic inhaler and a gray protective cap. Each carton contains one inhaler or two inhalers. Each canister contains 200 doses (puffs).
Not all pack sizes may be marketed.
Marketing authorization holder and manufacturer
Marketing authorization holder:
Glenmark Pharmaceuticals s.r.o.
Hvězdova 1716/2b
140 78 Prague 4
Czech Republic
Manufacturer:
Glenmark Pharmaceuticals Europe Limited
Building 2, Croxley Green Business Park
Croxley Green, WD18 8YA Hertfordshire
United Kingdom
Glenmark Pharmaceuticals s.r.o.
Fibichova 143
566 17 Vysoké Mýto
Czech Republic
Synoptis Industrial Sp. z o.o.
ul. Rabowicka 15
62-020 Swarzędz
Poland
To obtain more detailed information, contact the local representative of the marketing authorization holder:
Glenmark Pharmaceuticals Sp. z o.o.
ul. Osmańska 14
02-823 Warsaw
This medicinal product is authorized in the Member States of the European Economic Area under the following names:
| Country | Medicinal product name |
| United Kingdom | Soprobec 50/100/200/250 micrograms per actuation pressurised inhalation solution |
| Czech Republic | Soprobec |
| Denmark | Soprobec 50/100/200/250 micrograms per actuation pressurised inhalation solution |
| Finland | Soprobec 50/100/200/250 micrograms per actuation pressurised inhalation solution |
| Germany | Beclometason Glenmark Dosieraerosol 50/100/200/250 Mikrogramm/Sprühstoß Druckgasinhalation, Lösung |
| Italy | BECLOMETASONE DOC Generici |
| Netherlands | Soprobec 50/100/200/250 microgram/dosis aërosol, oplossing |
| Norway | Soprobec |
| Poland | Soprobec |
| Romania | Soprobec 50/100/200/250 micrograme pe doză soluţie de inhalat presurizată |
| Slovakia | Soprobec 50/100/200/250 micrograms per actuation pressurised inhalation solution |
| Spain | Soprobec 50/100/200/250 microgramos/inhalación, solución para inhalación en envase a presión |
| Sweden | Soprobec 50/100/200/250 micrograms/actuation pressurised inhalation, solution |
Date of last revision of the leaflet:December 2024
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- ImporterGlenmark Generics (Europe) Ltd Glenmark Pharmaceuticals s.r.o. Synoptis Industrial Sp. z o.o.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SoprobecDosage form: Aerosol, 100 mcg/measured doseActive substance: beclometasonePrescription requiredDosage form: Aerosol, 200 mcg/measured doseActive substance: beclometasonePrescription requiredDosage form: Aerosol, 250 mcg/measured doseActive substance: beclometasoneManufacturer: Glenmark Generics (Europe) Ltd Glenmark Pharmaceuticals s.r.o. Synoptis Industrial Sp. z o.o.Prescription required
Alternatives to Soprobec in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Soprobec in Ukraine
Alternative to Soprobec in Spain
Online doctors for Soprobec
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Soprobec – subject to medical assessment and local rules.














