
SOPROBEC 250 micrograms/inhalation pressurized solution for inhalation

How to use SOPROBEC 250 micrograms/inhalation pressurized solution for inhalation
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Leaflet: Information for the Patient
Soprobec 250 micrograms/inhalation inhalation solution in a pressurized container
Beclometasone, dipropionate
Read the entire leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any doubts, consult your doctor, pharmacist, or nurse.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor, pharmacist, or nurse, even if they are side effects not listed in this leaflet. See section 4.
Contents of the Leaflet
- What Soprobec is and what it is used for
- What you need to know before starting to use Soprobec
- How to use Soprobec
- Possible side effects
- Storage of Soprobec
- Contents of the pack and additional information
1. What Soprobec is and what it is used for
Soprobec inhalation solution in a pressurized container is used to help preventasthma symptoms. The active ingredient, beclometasone dipropionate, belongs to a group of medications known as corticosteroids, sometimes also called simply steroids. Steroids have an anti-inflammatory action, reducing swelling and irritation of the walls of the small airways in the lungs, making it easier to breathe.
2. What you need to know before starting to use Soprobec
Do not use Soprobec:
- if you are allergic to beclometasone dipropionate or to any of the components of this medication (listed in section 6) or any other medication used in the treatment of asthma.
- to treat a sudden attack of breathing difficulties. It will not help. Use a rapid-acting inhaler for this purpose and carry it with you at all times.
Warnings and Precautions
Consult your doctor, pharmacist, or nurse before starting to use Soprobec:
- if you have or have been treated for tuberculosis.
- if you have had thrush in your mouth
- you should avoid consuming alcohol for any reason
You should also consult your doctor, pharmacist, or nurse:
- if your asthma seems to be getting worse. You may be more breathless and wheezy than usual, your rescue inhaler seems to be less effective, you need more puffs than usual from your rescue inhaler, or you do not seem to be improving.
- when you are transferred from treatment with steroids in tablets to an inhaler, you may find that, even if your chest is improving, you feel unwell, develop a rash, or have a runny nose and sneezing (rhinitis). Do not stoptreatment with your inhaler unless your doctor tells you to.
If you have been treated with high doses for a long time with inhaled steroids, you may need treatment with steroids in tablets or possibly a steroid injection at times of stress. For example, during hospital admission after a serious accident, before an operation, during an acute asthma attack, or if you have a chest infection or other serious illness. Your doctor will consider whether you need supplementary steroid treatment and will inform you how long you need to continue treatment with steroid tablets and how to reduce them as you improve.
Contact your doctor if you experience blurred vision or other visual disturbances.
Children
Soprobec 250 micrograms/inhalation is not indicated in children.
Other Medications and Soprobec
Before starting treatment, inform your doctor if you are taking or have recently taken or may need to take any other medication, including those purchased without a prescription.
Inform your doctor if you are taking disulfiram or metronidazole, as there is a potential risk of interaction in particularly sensitive individuals.
Remember to carry these medications and your inhalers with you if you go to the hospital.
Some medications may increase the effects of Soprobec, so your doctor will monitor you closely if you are taking these medications (including some for HIV: ritonavir, cobicistat).
Pregnancy and Breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Driving and Using Machines
Soprobec has no known effects on driving or using machines.
Soprobec contains alcohol
This medication contains 8.62 mg of alcohol (ethanol) per inhalation, which is equivalent to 13%. The amount of alcohol in each inhalation is equivalent to less than 4 ml of beer or 2 ml of wine. The small amount of alcohol in this medication does not produce any noticeable effect.
3. How to use Soprobec
Soprobec is available in 4 different doses. Your doctor will decide which dose you need. Soprobec 200 micrograms/inhalation and Soprobec 250 micrograms/inhalation are not indicated for children.
Follow the administration instructions for this medication exactly as indicated by your doctor or pharmacist. If in doubt, consult your doctor or pharmacist again. The inhaler instructions are provided after the dosage section. The inhaler takes a few days to start working. It is very important that you use it regularly.
Do not stoptreatment even if you feel better, unless your doctor tells you to. Do not stopusing the inhaler suddenly.
During your treatment with Soprobec, your doctor will regularly assess your asthma through simple breathing tests and may need to perform occasional blood tests.
Dosage:
The initial dose will depend on the severity of your asthma and will be decided by your doctor. It may be higher than those described below. Your doctor will prescribe the lowest dose of Soprobec that controls your symptoms.
The Volumatic™ spacer chamber should always be used when:
- adults, elderly patients, and adolescents over 16 years old inhale total daily doses of Soprobec equal to or greater than 1,000 micrograms.
Soprobec 250 micrograms/inhalation
The standard initial dose is:
Only for adults and elderly patients: 500 micrograms (2 puffs) twice a day
Normally, the maximum you will inhale in a day is: 2,000 micrograms (8 puffs)
The total daily dose can be divided into 2, 3, or 4 daily doses.
This dose is not suitable for children
If you use more Soprobec than you should:
Let your doctor know as soon as possible. Your doctor may want to check your cortisol levels in your blood, so you will need to have a blood sample taken (cortisol is a human steroid hormone that is naturally present in the body).
It is important that you take your dose as indicated by your doctor. Do not increase or reduce your dose without medical supervision.
In case of overdose or accidental ingestion, consult the Toxicology Information Service. Phone: 91 562 04 20, consult your doctor or pharmacist.
If you forget to use Soprobec:
Do not take a double dose to make up for forgotten doses, simply wait for the next dose. Do not take more puffs than prescribed.
Method of Administration
Soprobec is for inhalation use only.
Instructions for Use:
It is essential that you know how to use your inhaler correctly. Your doctor, nurse, or pharmacist will teach you how to use your inhaler correctly and will periodically check that you are using it correctly. You must follow the instructions carefully, so you know how, when, and how manypuffs you should inhale and how often you should use your inhaler. If you are unsure what to do or have problems inhaling, consult your doctor, nurse, or pharmacist.
- To remove the protective cap, hold it between your index finger and thumb, squeeze gently, and pull the cap off as shown. Check that the mouthpiece is clean inside and out and that there are no foreign objects.
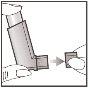
Inhaler Check:If the inhaler is new or has not been used for 3 or more days, perform a puff into the air to ensure it is working.
- Hold the inhaler in a vertical position as shown, with your thumb on the base, under the mouthpiece. Exhale as much as you can.
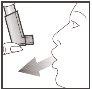
- Place the mouthpiece in your mouth between your teeth and close your lips around it, but do not bite.
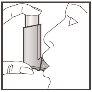
- Just after starting to inhale through your mouth, press the top of the inhaler to perform a puff while continuing to inhale deeply and evenly.
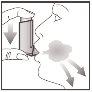
- Hold your breath; remove the inhaler from your mouth and your finger from the top of the inhaler. Continue holding your breath for a few seconds or as long as you can. Exhale slowly.
- If you need to perform another puff, hold the inhaler vertically and wait half a minute before repeating steps 2 to 5.
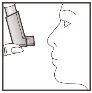
- After use, always replace the protective cap to prevent dust and fluff. Replace it firmly and insert it into its position.
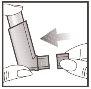
Important: Do not perform steps 2, 3, 4, and 5 quickly.
It is essential that you start inhaling as slowly as possible before operating the inhaler. Practice in front of a mirror the first few times.
If you see 'mist' coming out of the top of the inhaler or the sides of your mouth, the beclometasone will not reach your lungs properly. Take another puff, following the instructions carefully from step 2 onwards.
People with weak hands may find it easier to hold the inhaler with both hands. Put both index fingers on the top of the inhaler and both thumbs under the mouthpiece.
If you find it difficult to manage the inhaler while inhaling, use the Volumatic™ spacer chamber. Consult your doctor, nurse, or pharmacist about this device.
The Volumatic™ spacer chamber should always be used when:
- you are an adult, elderly patient, or adolescent over 16 years old who takes a total daily dose of Soprobec equal to or greater than 1,000 micrograms,
Tell your doctor, nurse, or pharmacist if you have difficulties.
Cleaning:
It is essential to clean the inhaler at least once a week to prevent it from becoming blocked.
- Remove the metal canister from the plastic casing and remove the protective cap.
- Rinse the plastic casing and protective cap with warm water. If you use a mild detergent, rinse carefully with clean water before drying. Do not put the metal canister in water.
- Let it dry well in a warm place. Avoid excessive heat.
- Replace the canister and protective cap.
It is essential that you read the leaflet that comes with the Volumatic™ spacer chamber and follow the instructions for use and cleaning of the Volumatic™.
4. Possible Side Effects
Like all medications, Soprobec can cause side effects, although not everyone will experience them.
Stop taking Soprobec and consult your doctor if:
- you experience hypersensitivity reactions such as skin rashes, hives, itching, or redness, or swelling of the face, eyes, lips, and throat.
- you notice a sudden increase in wheezing, difficulty breathing, and coughing after using your inhaler, stopusing Soprobec and use a rapid-acting rescue inhaler immediately. Contact your doctor immediately. Your doctor will assess your asthma and may change your treatment and prescribe a different inhaler for your asthma.
The following side effects have been reported. Tell your doctor as soon as possibleif you experience any of these side effects, but do not stop treatmentunless your doctor tells you to. Your doctor will try to prevent these effects by prescribing Soprobec at the lowest possible dose to control your asthma.
Very Common (may affect more than 1 in 10 people)
- Oral thrush (candidiasis). This is more likely if the dose is greater than 400 micrograms. Thrush can be treated with antifungals while you continue using Soprobec. Brushing your teeth or rinsing your mouth with water immediately after each dose may help prevent thrush.
Common (may affect up to 1 in 10 people)
- Hoarseness or throat irritation. Using a Volumatic™ spacer chamber for inhalation or rinsing your mouth with water immediately after each dose may help.
Rare (may affect up to 1 in 100 people)
- Rashes, hives, itching, and/or redness.
Very Rare (may affect up to 1 in 10,000 people)
- Allergic reactions, which manifest as swelling of the eyelids, face, lips, and/or throat (angioedema).
- Respiratory disorders such as dyspnea (feeling of lack of air or difficulty breathing) and/or bronchospasm (narrowing of the bronchial walls with decreased air intake).
- Anaphylactic or anaphylactoid reactions (severe allergic reactions that can make breathing difficult and alter your level of consciousness).
- Delayed growth in children and adolescents, who may need to have their height checked periodically by their doctor. This occurs particularly after treatment with Soprobec at high doses for a long time.
- Round face (moon face) (Cushing's syndrome).
- Loss of bone density (thinning and weakening of bones)
- Eye problems such as cataract formation or glaucoma (increased pressure in the eye).
- Paradoxical bronchospasm.
Frequency Not Known:
- Sleep problems, depression, or feelings of worry, anxiety, nervousness, overexcitement, or irritability. These effects are more likely to occur in children.
- Blurred vision.
- Headache.
- Nausea.
If you become illor develop symptoms such as loss of appetite, abdominal pain, weight loss, fatigue, nausea (feeling unwell), vomiting, feeling faint, sweating, and possible convulsions (seizures), you should consult your doctor. This is particularly important if you have been exposed to stressful situations such as surgery, infection, an acute asthma attack, or other serious illness. Your doctor may perform a blood test to monitor the levels of steroids in your body.
Reporting of Side Effects
If you experience any side effects, consult your doctor or pharmacist, even if they are side effects not listed in this leaflet. You can also report them directly through the Spanish Medication Surveillance System for Human Use: http://www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Soprobec
Keep this medication out of the sight and reach of children.
Do not use this medication after the expiration date stated on the outer packaging and label, after the EXP date. The expiration date is the last day of the month indicated.
Do not freeze.
Store in the original packaging to protect it from light.
If the inhaler becomes very cold, remove the metal canister from the plastic casing and warm it in your handsfor a few minutes before use. Neveruse anything else to heat it.
Medications should not be disposed of through wastewater or household waste. Deposit the packaging and any unused medications in the pharmacy's SIGRE collection point. Ask your pharmacist how to dispose of the packaging and any unused medications. This will help protect the environment.
Warning:The container contains a pressurized liquid. Keep away from heat and direct sunlight, do not expose to high temperatures (above 50°C), and do not puncture or burn (incinerate), even when empty.
6. Container Content and Additional Information
Composition of Soprobec
- The active principle of your inhaler is beclometasone dipropionate. Each puff contains 250 micrograms of beclometasone dipropionate.
- The other excipients are glycerol, anhydrous ethanol, and norflurane (HFC-134a).
This medication contains fluorinated greenhouse gases.
Each inhaler contains 11,484 g of HFC-134a, which corresponds to 0,016 tons of CO2 equivalent (global warming potential = 1430).
Appearance of Soprobec and Container Content
Soprobec 250 micrograms/inhalation:
Soprobec is an inhalation solution in a pressurized aluminum container with a light burgundy plastic actuator and a gray protective cap. Each container contains a single inhaler or two inhalers. Each container contains 200 puffs.
Not all pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
Glenmark Arzneimittel GmbH
Industriestr. 31
82194 Grobenzell
Germany
Manufacturer:
Glenmark Pharmaceuticals s.r.o.
Fibichova 143
56617 Vysoke Myto
Czech Republic
Glenmark Pharmaceuticals Europe Limited
Building 2, Croxley Green Business Park, Croxley Green
Hertfordshire WD18 8YA
United Kingdom
Synoptis Industrial Sp. z.o.o.
Ul. Rabowicka 15
62-020 Swarzedz
Poland
You can request more information about this medication by contacting the local representative of the Marketing Authorization Holder:
Glenmark Farmacéutica, S.L.U.
C/ Retama 7, 7th floor
28045 Madrid
Spain
This medication is authorized in the Member States of the European Economic Area under the following names:
Czech Republic | Soprobec |
Germany | Beclometason Glenmark Dosieraerosol 250 Mikrogramm/Sprühstoß Druckgasinhalation, Lösung |
Italy | Soprobec |
Netherlands | Soprobec 250 microgram/dose aerosol, solution |
Poland | Soprobec |
Romania | Soprobec 250 micrograms/dose pressurized inhalation solution |
Spain | Soprobec 250 micrograms/inhalation, pressurized inhalation solution |
Date of the last revision of this prospectus:December 2024.
Detailed information about this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://aemps.gob.es/.
Volumatic™ is a trademark of GlaxoSmithKline Group of Companies.
- Country of registration
- Average pharmacy price17.48 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SOPROBEC 250 micrograms/inhalation pressurized solution for inhalationDosage form: PULMONARY INHALATION, 100 micrograms/actuationActive substance: beclometasoneManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: PULMONARY INHALATION, 50 micrograms/actuationActive substance: beclometasoneManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: PULMONARY INHALATION, 250 µg beclomethasone dipropionateActive substance: beclometasoneManufacturer: Glaxosmithkline S.A.Prescription required
Online doctors for SOPROBEC 250 micrograms/inhalation pressurized solution for inhalation
Discuss questions about SOPROBEC 250 micrograms/inhalation pressurized solution for inhalation, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















