
BECLO-ASMA 50 micrograms/actuation pressurized inhalation solution

Ask a doctor about a prescription for BECLO-ASMA 50 micrograms/actuation pressurized inhalation solution

How to use BECLO-ASMA 50 micrograms/actuation pressurized inhalation solution
Introduction
Leaflet: Information for the patient
Beclo-Asma 50 micrograms/puff inhalation solution in pressurized container
beclometasone dipropionate
Read this leaflet carefully before starting to use this medication, as it contains important information for you.
- Keep this leaflet, as you may need to read it again.
- If you have any questions, consult your doctor or pharmacist.
- This medication has been prescribed to you only, and you should not give it to others, even if they have the same symptoms as you, as it may harm them.
- If you experience side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. See section 4.
Contents of the leaflet
- What is Beclo-Asma 50 micrograms/puff and what is it used for
- What you need to know before starting to use Beclo-Asma 50 micrograms/puff
- How to use Beclo-Asma 50 micrograms/puff
- Possible side effects
- Storage of Beclo-Asma 50 micrograms/puff
- Container contents and additional information
1. What is Beclo-Asma 50 micrograms/puff and what is it used for
Beclo-Asma 50 micrograms/puff contains the active ingredient beclometasone dipropionate, which belongs to the group of medications called corticosteroids. Corticosteroids are used to treat asthma because they have, among other effects, an anti-inflammatory action. They reduce swelling and irritation in the walls of the small airways in the lungs and make breathing easier.
Beclo-Asma is used to prevent asthma symptoms in people who need regular treatment.
Corticosteroids also help prevent asthma attacks.
It is not indicated for the relief of acute bronchospasm.
2. What you need to know before starting to use Beclo-Asma 50 micrograms/puff
Do not use Beclo-Asma 50 micrograms/puff:
- If you are allergic to beclometasone dipropionate or any of the other components of this medication (listed in section 6).
- To treat a sudden attack of breathing difficulty.
Warnings and precautions
Consult your doctor or pharmacist before starting to use Beclo-Asma 50 micrograms/puff:
- If you have had oral thrush
- If you are taking or have recently taken any type of steroid tablet or injection
- If you are being or have been treated for tuberculosis.
- If you have a history of diabetes mellitus (as this medication may increase blood glucose levels),
- If you have used high doses of this medication for a prolonged period and experience the following symptoms:
- Weight gain and rounding of the face (moon face) (Cushing's syndrome),
- Vague symptoms, such as abdominal pain, nausea, diarrhea, headache, or drowsiness (adrenal suppression, acute adrenal crisis). These symptoms are more likely during an infection, such as viral infections or stomach upset,
- Bone loss,
- Eye problems (cataracts and glaucoma),
- Growth retardation (this occurs mainly in children and adolescents).
Contact your doctor if you experience blurred vision or other visual disturbances.
Patients who have previously been treated with beclometasone should be aware that this medication does not contain chlorofluorocarbons (CFCs). The active ingredient is the same. The only differences that may be noticed are the taste and sensation of the spray in the mouth, as well as the sound of the inhaler during use.
It should be used exactly as your doctor indicates. Your doctor may change the dosage regimen.
Use in athletes
Athletes are informed that this medication contains beclometasone dipropionate, which may result in a positive doping test.
Other medications and Beclo-Asma 50
Tell your doctor or pharmacist if you are taking, have recently taken, or may take any other medication.
Some medications may increase the effects of Beclo-Asma 50 micrograms/puff, so your doctor will monitor you closely if you are taking these medications (including some for HIV: ritonavir, cobicistat).
Tell your doctor if you are taking disulfiram or metronidazole, as there is a potential risk of interaction in particularly sensitive individuals.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medication.
Pregnancy: there is insufficient evidence on the use of the product during pregnancy.
The use of beclometasone dipropionate during pregnancy requires weighing the potential benefits of the medication against the possible risks.
Breastfeeding: it is likely that beclometasone is excreted in breast milk. However, considering the relatively low doses used via inhalation, it is likely that the levels are low. In breastfeeding mothers, the therapeutic benefits of the medication should be weighed against the potential risks to the mother and baby.
Driving and using machines
Beclo-Asma 50 micrograms/puff has no known effects on driving or using machines.
Beclo-Asma 50 micrograms/puff contains ethanol
This medication contains approximately 4.7 mg of alcohol (ethanol) per inhalation. The amount per inhalation of this medication is equivalent to less than 1 ml of beer or 1 ml of wine. The small amount of alcohol in this medication does not produce any noticeable effect.
.
3. How to use Beclo-Asma 50 micrograms/puff
Follow your doctor's instructions for administering this medication exactly. If you have any doubts, consult your doctor or pharmacist again.
Remember to use your medication.
Beclo-Asma should only be used by inhalation.
Your doctor will indicate the duration of your treatment with Beclo-Asma. Do not stop treatment before, even if you feel better, unless your doctor tells you to or you notice that your breathing worsens when taking the medication. The usual recommended doses are as follows:
Adults and children over 12 years
The initial dose for mild or moderate asthma is one inhalation (50 micrograms) twice a day. This dose may be increased to two inhalations (100 micrograms) twice a day.
For more severe asthma, the usual dose is up to four inhalations (200 micrograms) twice a day.
The maximum recommended dose is 800 micrograms per day.
Children from 6 to 11 years
The dose should be determined by the doctor and depends on the individual response of each patient. In general, it is recommended to use half the doses recommended for adults (maximum 400 micrograms per day in two divided doses). Children should receive an initial dose of Beclo-Asma suitable for the severity of their disease. The dose can then be adjusted until adequate control is achieved or reduced to the minimum effective dose according to individual response.
If you start using Beclo-Asma, instead of, or at the same time as, oral steroids, you should carry a warning card stating that you are taking steroids until your doctor tells you that you no longer need it.
If you have difficulties or do not understand the instructions, consult your doctor or pharmacist.
It may take several days before you notice the benefits of the medication. It is very important to use it regularly every day. Do not interrupt treatment unless your doctor tells you to or you notice that your breathing worsens when taking the medication.
Do not use this medication to treat a sudden attack of breathing difficulty; it will not help. You will need a different type of medication.
Instructions for correct administration of the preparation:
Beclo-Asma 50 micrograms/puff is administered by inhalation. The correct handling of this medication is crucial for the success of the treatment.
Before each application, the following guidelines should be observed:
- Remove the protective cap (fig. 1). If it is a new inhaler or has not been used for a week, shake the container (fig. 2) and perform two puffs to ensure the inhaler is working properly. If the inhaler is used regularly, proceed to the next instructions:
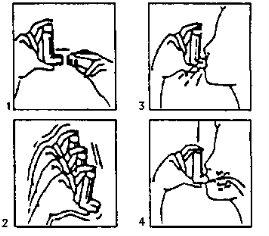 Shake the inhaler
Shake the inhaler- Remove as much air as possible from your lungs.
- Attach the inhaler to your mouth according to the position indicated in the drawing (fig. 3).
- Take a deep breath. You should press, according to the arrows in the drawing (fig. 4), the device while taking this breath.
- Remove the inhaler from your mouth and try to hold your breath for a few seconds, then exhale slowly.
- You should periodically wash the plastic mouthpiece. To do this, remove it from the aerosol and rinse it with plenty of water at least once a week. Before use, it should be dry. Do not wet the metal cartridge.
- Store with the protective cap in place to protect it from dust and dirt.
- It is recommended to rinse your mouth with water after each inhalation.
If you think the effect of Beclo-Asma 50 micrograms/puff is too strong or too weak, tell your doctor or pharmacist.
If you use more Beclo-Asma 50 micrograms/puff than you should
In case of overdose or accidental ingestion, consult your doctor or pharmacist immediately, or call the Toxicology Information Service, phone 915620420, indicating the medication and the amount taken.
If you forget to take Beclo-Asma 50 micrograms/puff
Do not take a double dose to make up for forgotten doses; simply wait for the next dose.
If you have any other questions about the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medications, this medication can cause side effects, although not everyone will experience them.
The following side effects have been reported:
-Common (may affect up to 1 in 10 people): oral and/or pharyngeal candidiasis (thrush), hoarseness and/or throat irritation, taste disturbances.
-Uncommon (may affect up to 1 in 100 people): headache, dizziness, tremors, cough, increased asthma symptoms, nausea, urticaria, itching, pruritus, erythema, purpura.
-Rare (may affect up to 1 in 1,000 people): allergic reactions, angioedema in eyes, throat, lips, and face, paradoxical bronchospasm.
-Very rare (may affect up to 1 in 10,000 people): adrenal suppression, growth retardation in children and adolescents, cataracts, glaucoma, decreased bone mineral density.
-Frequency not known (cannot be estimated from available data): psychomotor hyperactivity, sleep disorders, anxiety, depression, aggression, changes in behavior (commonly in children), and blurred vision.
Reporting side effects
If you experience any side effects, consult your doctor or pharmacist, even if they are not listed in this leaflet. You can also report them directly through the Spanish Medication Surveillance System for Human Use: https://www.notificaram.es. By reporting side effects, you can help provide more information on the safety of this medication.
5. Storage of Beclo-Asma 50 micrograms/puff
Keep this medication out of the sight and reach of children.
Protect from direct sunlight. Do not freeze.
If the inhaler is very cold, remove the cartridge and warm it with your hand for a few minutes before use. Do not use any other method to heat it.
The container contains a pressurized liquid. Do not expose to temperatures above 50°C. Do not puncture the container, even if it appears to be empty.
Do not use this medication after the expiration date stated on the container after Cad.:. The expiration date is the last day of the month indicated.
Medications should not be disposed of through wastewater or household waste. Deposit the containers and medications you no longer need in the SIGRE collection point at the pharmacy. If you have any doubts, ask your pharmacist how to dispose of the containers and medications you no longer need. This will help protect the environment.
6. Container contents and additional information
Composition of Beclo-Asma 50 micrograms/puff:
- The active ingredient is beclometasone dipropionate.
- The other components (excipients) are anhydrous ethanol and Norflurane.
Each inhalation contains 50 micrograms of beclometasone dipropionate.
Appearance of the product and container contents:
Beclo-Asma 50 micrograms/puff is a clear, colorless solution presented in a 10 ml container (200 applications) with a dosing valve and oral adapter.
Marketing authorization holder and manufacturer:
Laboratorio Aldo-Unión, S.L.
Baronesa de Maldá, 73
08950 Esplugues de Llobregat
Barcelona – Spain
Date of the last revision of this leaflet:10/2017
Detailed and updated information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://www.aemps.es/
- Country of registration
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to BECLO-ASMA 50 micrograms/actuation pressurized inhalation solutionDosage form: PULMONARY INHALATION, 100 micrograms/actuationActive substance: beclometasoneManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: PULMONARY INHALATION, 250 µg beclomethasone dipropionateActive substance: beclometasoneManufacturer: Glaxosmithkline S.A.Prescription requiredDosage form: PULMONARY INHALATION, 50 mcg beclomethasone dipropionateActive substance: beclometasoneManufacturer: Glaxosmithkline S.A.Prescription required
Alternatives to BECLO-ASMA 50 micrograms/actuation pressurized inhalation solution in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to BECLO-ASMA 50 micrograms/actuation pressurized inhalation solution in Poland
Alternative to BECLO-ASMA 50 micrograms/actuation pressurized inhalation solution in Ukraine
Online doctors for BECLO-ASMA 50 micrograms/actuation pressurized inhalation solution
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for BECLO-ASMA 50 micrograms/actuation pressurized inhalation solution – subject to medical assessment and local rules.














