
SOPROBEC 50 micrograms/inhalation pressurized solution for inhalation

How to use SOPROBEC 50 micrograms/inhalation pressurized solution for inhalation
Translated with AI
This page provides general information and does not replace a doctor’s consultation. Always consult a doctor before taking any medication. Seek urgent medical care if symptoms are severe.
Show originalContents of the leaflet
Introduction
Package Leaflet: Information for the Patient
Soprobec 50 micrograms/inhalation inhalation solution in a pressurized container
Beclometasone, dipropionate
Read this package leaflet carefully before you start using this medicine, because it contains important information for you.
- Keep this package leaflet, you may need to read it again.
- If you have any further questions, ask your doctor, pharmacist, or nurse.
- This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If you experience any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this package leaflet. See section 4.
Contents of the package leaflet
- What is Soprobec and what is it used for
- What you need to know before you start using Soprobec
- How to use Soprobec
- Possible side effects
- Storage of Soprobec
- Contents of the pack and further information
1. What is Soprobec and what is it used for
Soprobec inhalation solution in a pressurized container is used to help preventasthma symptoms. The active ingredient, beclometasone dipropionate, belongs to a group of medicines known as corticosteroids, sometimes also simply called steroids. Steroids have an anti-inflammatory action, reducing swelling and irritation of the walls of the small airways in the lungs, making it easier to breathe.
2. What you need to know before you start using Soprobec
Do not use Soprobec:
- if you are allergic to beclometasone dipropionate or any of the other ingredients of this medicine (listed in section 6) or to any other medicine used to treat asthma.
- to treat a sudden attack of difficulty breathing. It will not help. Use a fast-acting reliever inhaler for this purpose and carry it with you at all times.
Warnings and precautions
Talk to your doctor, pharmacist, or nurse before you start using Soprobec:
- if you have been treated for tuberculosis.
- if you have had thrush in your mouth.
- you should avoid drinking alcohol for any reason.
You should also talk to your doctor, pharmacist, or nurse:
- if your asthma seems to be getting worse. You may be more breathless than usual, your reliever inhaler may be less effective, you may need more puffs than usual from your reliever inhaler, or you may not seem to be getting better.
- when you are transferred from systemic steroid treatment to an inhaler, you may find that, even if your chest is improving, you feel unwell, develop a rash, itching, or sneezing (rhinitis). Do not stopyour inhaler treatment unless your doctor tells you to.
If you have been treated with high doses of inhaled steroids for a long time, you may need steroid treatment in tablet form or possibly a steroid injection at times of stress. For example, during hospital admission after a serious accident, before an operation, during a severe asthma attack, or if you have a chest infection or other serious illness. Your doctor will consider whether you need additional steroid treatment and will inform you how long you need to continue tablet steroid treatment and how to reduce it as you get better.
Contact your doctor if you experience blurred vision or other visual disturbances.
Other medicines and Soprobec
Before you start treatment, tell your doctor if you are taking or have recently taken or might take any other medicines, including those obtained without a prescription.
Tell your doctor if you are taking disulfiram or metronidazole, as there is a potential risk of interaction in particularly sensitive individuals.
Remember to carry these medicines and your inhalers with you if you go to the hospital.
Some medicines may increase the effects of Soprobec, so your doctor will monitor you closely if you are taking these medicines (including some for HIV: ritonavir, cobicistat).
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or plan to become pregnant, consult your doctor or pharmacist before using this medicine.
Driving and using machines
Soprobec has no known effects on driving or using machines.
Soprobec contains alcohol
This medicine contains 7.47 mg of alcohol (ethanol) per inhalation, which is equivalent to 13%. The amount of alcohol in each inhalation is equivalent to less than 4 ml of beer or 2 ml of wine. The small amount of alcohol in this medicine does not produce any noticeable effect.
3. How to use Soprobec
Soprobec is available in 4 different doses. Your doctor will decide which dose you need. Soprobec 200 micrograms/inhalation and Soprobec 250 micrograms/inhalation are not indicated for children.
Follow the instructions for administration of this medicine exactly as told by your doctor or pharmacist. If you are unsure, consult your doctor or pharmacist again. The instructions for use of the inhaler are given after the dosage section. The inhaler takes a few days to start working. It is very important that you use it regularly.
Do not stoptreatment even if you feel better, unless your doctor tells you to. Do not stopusing the inhaler suddenly.
During your treatment with Soprobec, your doctor will regularly assess your asthma through simple breathing tests and may need to perform occasional blood tests.
Dosage:
The initial dose will depend on the severity of your asthma and will be decided by your doctor. It may be higher than those described below. Your doctor will prescribe the lowest dose of Soprobec that controls your symptoms.
The Volumatic spacer device should always be used when:
- adults, elderly patients, and adolescents over 16 years inhale total daily doses of Soprobec equal to or greater than 1,000 micrograms.
- when Soprobec is used in children and adolescents under 15 years at any prescribed dose.
Soprobec 50 micrograms/inhalation
The standard initial dose is:
Adults and elderly patients: 200 micrograms (4 puffs) twice daily
Children: 100 micrograms (2 puffs) twice daily
Normally, the maximum you will inhale in a day is:
Adults and elderly patients: 800 micrograms (16 puffs)
Children: 400 micrograms (8 puffs)
The total daily dose can be divided into 2, 3, or 4 daily doses.
If you use more Soprobec than you should:
Tell your doctor as soon as possible. Your doctor may want to check your cortisol levels in your blood, so you will need to have a blood test (cortisol is a human steroid hormone that is naturally present in the body).
It is important that you take your dose as instructed by your doctor. Do not increase or reduce your dose without medical supervision.
In case of overdose or accidental ingestion, contact the Toxicological Information Service. Phone: 91 562 04 20, consult your doctor or pharmacist.
If you forget to use Soprobec:
Do not take a double dose to make up for forgotten doses, just wait for the next dose. Do not take more puffs than prescribed.
Method of administration
Soprobec is for inhalation use only.
Instructions for use:
It is important that you know how to use your inhaler correctly. Your doctor, nurse, or pharmacist will teach you how to use your inhaler correctly and will check periodically that you are using it correctly. You must follow the instructions carefully, so you know how, when, and how manypuffs to inhale and how often to use your inhaler. If you are unsure what to do or have problems inhaling, consult your doctor, nurse, or pharmacist.
- To remove the protective cap, hold it between your thumb and index finger, squeeze gently, and pull the cap off as shown. Check that the mouthpiece is clean inside and outside and that there are no foreign objects.
Inhaler check:If the inhaler is new or has not been used for 3 or more days, perform a puff into the air to make sure it is working.
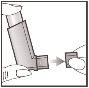
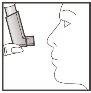
- Hold the inhaler in a vertical position as shown, with your thumb on the base, below the mouthpiece. Breathe out as much as you can.
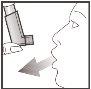
- Place the mouthpiece in your mouth between your teeth and close your lips around it, but do not bite.
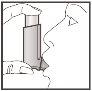
- Just after you start breathing in through your mouth, press the top of the inhaler to release a puff while continuing to breathe in deeply and evenly.
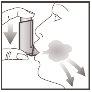
- Hold your breath; remove the inhaler from your mouth and your finger from the top of the inhaler. Continue holding your breath for a few seconds or as long as you can. Breathe out slowly.
- If you need to take another puff, hold the inhaler vertically and wait for half a minute before repeating steps 2 to 5.
- After use, always replace the protective cap to avoid dust and fluff. Replace it firmly and insert it into its position.
Important: Do not perform steps 2, 3, 4, and 5 quickly.
It is important that you start breathing in as slowly as possible before operating the inhaler. Practice in front of a mirror the first few times.
If you see 'mist' coming out of the top of the inhaler or the sides of your mouth, the beclometasone will not reach your lungs properly. Take another puff, following the instructions carefully from step 2 onwards.
People with weak hands or children may find it easier to hold the inhaler with both hands. Put both index fingers on the top of the inhaler and both thumbs below the mouthpiece.
If you find it difficult to manage the inhaler while inhaling, use the Volumatic spacer device. Consult your doctor, nurse, or pharmacist about this device.
The Volumatic spacer device should always be used when:
- you are an adult, elderly patient, or adolescent over 16 years who takes a total daily dose of Soprobec equal to or greater than 1,000 micrograms,
- Soprobec is used in children and adolescents under 15 years at any prescribed dose.
Young children may have difficulty using the inhaler and may need help. Using the inhaler with the Volumatic spacer device and a mask can help children under 5 years. Tell your doctor, nurse, or pharmacist if you have difficulties.
Cleaning:
It is important to clean the inhaler at least once a week to avoid blockage.
- Remove the metal canister from the plastic casing and remove the protective cap.
- Rinse the plastic casing and protective cap with warm water. If you use a mild detergent, rinse carefully with clean water before drying. Do not put the metal canister in water.
- Let it dry well in a warm place. Avoid excessive heat.
- Replace the canister and protective cap.
It is important that you read the package leaflet that comes with the Volumatic spacer device and follow the instructions for use and cleaning of the Volumatic.
4. Possible side effects
Like all medicines, Soprobec can cause side effects, although not everybody gets them.
Stop taking Soprobec and consult your doctor if:
- you suffer from hypersensitivity reactions such as skin rashes, hives, itching, or redness, or swelling of the face, eyes, lips, and throat.
- you notice a sudden increase in wheezing, difficulty breathing, and coughing after using your inhaler, stopusing Soprobec and use a fast-acting reliever inhaler immediately. Contact your doctor immediately. Your doctor will assess your asthma and may change your treatment and prescribe a different inhaler for your asthma.
The following side effects have been reported. Tell your doctor as soon as possibleif you experience any of these side effects, but do not stoptreatment unless your doctor tells you to. Your doctor will try to prevent these effects by prescribing Soprobec at the lowest dose possible to control your asthma.
Very common (may affect more than 1 in 10 people)
- Oral candidiasis (thrush). This is more likely if the dose is greater than 400 micrograms. Thrush can be treated with antifungals while you continue using Soprobec. Rinsing your mouth with water immediately after each dose may help prevent thrush.
Common (may affect up to 1 in 10 people)
- Hoarseness or throat irritation. Using a Volumatic spacer device or rinsing your mouth with water immediately after each dose may help.
Rare (may affect up to 1 in 100 people)
- Rashes, hives, itching, and/or redness.
Very rare (may affect up to 1 in 10,000 people)
- Allergic reactions, which manifest as swelling of the eyelids, face, lips, and/or throat (angioedema).
- Respiratory disorders such as dyspnea (feeling of shortness of breath or difficulty breathing) and/or bronchospasm (narrowing of the bronchial walls with decreased air intake).
- Anaphylactic or anaphylactoid reactions (severe allergic reactions that can make breathing difficult and alter your level of consciousness).
- Delayed growth in children and adolescents; they may need to have their height checked periodically by their doctor. This occurs particularly after treatment with Soprobec at high doses for a long period.
- Round face (moon face) (Cushing's syndrome).
- Loss of bone density (thinning and weakening of bones).
- Eye problems such as cataract formation or glaucoma (increased pressure in the eye).
- Paradoxical bronchospasm.
Frequency not known:
- Sleep problems, depression, or feelings of worry, anxiety, nervousness, overexcitement, or irritability. These effects are more likely to occur in children.
- Blurred vision.
- Headache.
- Nausea.
If you become unwellor develop symptoms such as loss of appetite, abdominal pain, weight loss, fatigue, nausea (feeling of discomfort), vomiting, feeling of fainting, sweating, and possible convulsions (seizures), you should consult your doctor. This is particularly important if you have been exposed to stressful situations such as surgery, infection, a severe asthma attack, or other serious illness. Your doctor may perform a blood test to monitor the levels of steroids in your body.
Reporting of side effects
If you experience any side effects, talk to your doctor or pharmacist. This includes any possible side effects not listed in this package leaflet. You can also report side effects directly through the Spanish Pharmacovigilance System for Human Use Medicines: http://www.notificaRAM.es. By reporting side effects, you can help provide more information on the safety of this medicine.
5. Storage of Soprobec
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date stated on the outer packaging and label, after EXP. The expiry date is the last day of the month stated.
Do not freeze.
Store in the original packaging to protect from light.
If the inhaler becomes very cold, remove the metal canister from the plastic casing and warm it in your handsfor a few minutes before use. Neveruse anything else to warm it up.
Medicines should not be disposed of via wastewater or household waste. Return any unused medicine to a pharmacy for disposal. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
Warning:The container contains a pressurized liquid. Keep away from heat and direct sunlight, do not expose to high temperatures (above 50°C), and do not pierce or burn (incinerate), even when empty.
6. Container Contents and Additional Information
Soprobec Composition
- The active ingredient in your inhaler is beclometasone dipropionate. Each actuation contains 50 micrograms of beclometasone dipropionate.
- The other excipients are glycerol, anhydrous ethanol, and norflurane (HFC-134a).
This medication contains fluorinated greenhouse gases.
Each inhaler contains 11.808 g of HFC-134a, which corresponds to 0.017 tons of CO2 equivalent (global warming potential = 1430).
Appearance of Soprobec and Container Contents
Soprobec 50 micrograms/inhalation:
Soprobec is an inhalation solution in a pressurized aluminum container with a cream-colored plastic actuator and a dark brown protective cap. Each container contains a single inhaler or two inhalers. Each container contains 200 actuations.
Not all pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorization Holder:
Glenmark Arzneimittel GmbH
Industriestr. 31
82194 Grobenzell
Germany
Manufacturer:
Glenmark Pharmaceuticals s.r.o.
Fibichova 143
56617 Vysoke Myto
Czech Republic
Glenmark Pharmaceuticals Europe Limited
Building 2, Croxley Green Business Park, Croxley Green
Hertfordshire WD18 8YA
United Kingdom
Synoptis Industrial Sp. z.o.o.
Ul. Rabowicka 15
62-020 Swarzedz
Poland
You can request more information about this medication by contacting the local representative of the Marketing Authorization Holder:
Glenmark Farmacéutica, S.L.U.
C/ Retama 7, 7ª planta
28045 Madrid
Spain
This medication is authorized in the Member States of the European Economic Area under the following names:
Czech Republic | Soprobec |
Germany | Beclometason Glenmark Dosieraerosol 50 Mikrogramm/Sprühstoß Druckgasinhalation, Lösung |
Italy | Soprobec |
Netherlands | Soprobec 50 microgram/dose aerosol, solution |
Poland | Soprobec |
Romania | Soprobec 50 micrograms/dose pressurized inhalation solution |
Spain | Soprobec 50 micrograms/inhalation, pressurized inhalation solution |
Date of the last revision of this leaflet:January 2021.
Detailed information on this medication is available on the website of the Spanish Agency for Medicines and Health Products (AEMPS) http://aemps.gob.es/.
Volumatic™ is a trademark of GlaxoSmithKline Group of Companies.
- Country of registration
- Average pharmacy price3.5 EUR
- Active substance
- Prescription requiredYes
- Manufacturer
- This information is for reference only and does not constitute medical advice. Always consult a doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to SOPROBEC 50 micrograms/inhalation pressurized solution for inhalationDosage form: PULMONARY INHALATION, 100 micrograms/actuationActive substance: beclometasoneManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: PULMONARY INHALATION, 50 micrograms/actuationActive substance: beclometasoneManufacturer: Laboratorio Aldo Union S.L.Prescription requiredDosage form: PULMONARY INHALATION, 250 µg beclomethasone dipropionateActive substance: beclometasoneManufacturer: Glaxosmithkline S.A.Prescription required
Online doctors for SOPROBEC 50 micrograms/inhalation pressurized solution for inhalation
Discuss questions about SOPROBEC 50 micrograms/inhalation pressurized solution for inhalation, including use, safety considerations and prescription review, subject to medical assessment and local regulations.
Frequently Asked Questions















