
Riastap

Ask a doctor about a prescription for Riastap

How to use Riastap
INFORMATION LEAFLET: INFORMATION FOR THE PATIENT
Riastap, 1 g
Powder for solution for injection/infusion.
Human fibrinogen
Please read carefully the contents of this leaflet before using the medicine, as it contains important information for the patient.
- Please keep this leaflet, so you can read it again if you need to.
- In case of any doubts, you should consult a doctor or pharmacist.
- This medicine has been prescribed specifically for you. Do not pass it on to others. The medicine may harm another person, even if their symptoms are the same.
- If the patient experiences any side effects, including any possible side effects not listed in the leaflet, they should inform their doctor or pharmacist. See section 4.
Table of contents of the leaflet:
- 1. What is Riastap and what is it used for
- 2. Important information before using Riastap
- 3. How to use Riastap
- 4. Possible side effects
- 5. How to store Riastap
- 6. Contents of the pack and other information
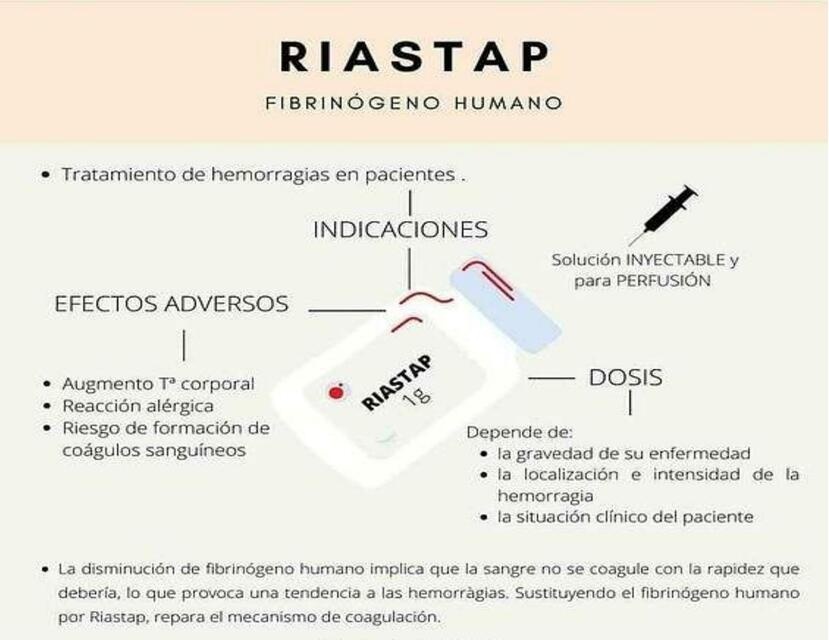
1. What is Riastap and what is it used for
What is Riastap?
Riastap contains human fibrinogen, which is an essential protein for blood clotting (coagulation). A lack of fibrinogen means that the blood does not clot as quickly as it should, which can lead to an increased tendency to bleed. Replacing human fibrinogen with Riastap medicine repairs the clotting mechanisms.
What is Riastap used for?
Riastap is used to treat bleeding in patients with a congenital lack of fibrinogen (hypo- or afibrinogenemia) with a tendency to bleed.
2. Important information before using Riastap
Information provided in this section should be taken into account by the doctor before administering the Riastap medicine.
When not to use Riastap:
- if the patient is allergic to human fibrinogen or to any of the other ingredients of this medicine (listed in section 6).
In case of allergy to any medicine or food, the doctor should be informed.
Warnings and precautions:
- if there have been allergic reactions to Riastap in the past. In this case, prophylactic antihistamines and corticosteroids should be taken, if recommended by the doctor.
- when allergic reactions or anaphylactic reactions (severe allergic reaction that causes severe breathing difficulties or dizziness) occur. Administration
Riastap should be stopped immediately (e.g., by stopping the injection).
- due to the increased risk of blood clot formation in a blood vessel (thrombosis), in particular:
- in case of high doses or repeated dosing
- when there is a history of heart disease (coronary heart disease or myocardial infarction)
- in case of liver disease
- immediately after surgery (post-operative patients)
- shortly before surgery (pre-operative patients)
- in newborn babies (neonates)
- if there is a higher than normal tendency to form blood clots (patients with a risk of thromboembolic disorders or disseminated intravascular coagulation)
The doctor will consider the benefit of therapy with Riastap compared to the risk associated with the above complications.
Viral safety
In the case of medicinal products obtained from human blood or plasma, measures are taken to prevent infections from being transmitted to patients.
These include:
- careful selection of blood and plasma donors to exclude infected individuals and
- testing of individual donations and plasma pools for the presence of viruses/infections.
Manufacturers of these products also include steps in the blood or plasma processing process that can inactivate or eliminate viruses. Despite this, it is not possible to completely exclude the possibility of transmitting infectious agents when administering medicinal products obtained from human blood or plasma. This also applies to unknown and newly discovered viruses and other types of infections.
These procedures are considered effective against enveloped viruses, such as human immunodeficiency virus (HIV, the virus that causes AIDS), hepatitis B and C viruses (inflammation of the liver), as well as against non-enveloped viruses such as hepatitis A virus (inflammation of the liver) and parvovirus B19.
The doctor may recommend considering vaccination against viral hepatitis A and B in case of regular/repeated administration of products derived from human plasma.
It is recommended that the doctor records the date of administration of the preparation, batch number, and injected volume.
Riastap and other medicines
- The doctor or pharmacist should be informed about all medicines currently being taken by the patient or recently taken, as well as medicines that the patient plans to take.
- Riastap medicine should not be mixed with other medicinal products, except as described in the section "Information intended exclusively for healthcare professionals / Reconstitution".
Pregnancy and breastfeeding
- If the patient is pregnant or breastfeeding, thinks they may be pregnant, or plans to have a child, they should consult a doctor or pharmacist before using this medicine.
- During pregnancy or breastfeeding, Riastap preparation should only be administered if clearly necessary.
Driving and using machines
Riastap does not affect or has negligible effect on the ability to drive vehicles or operate machines.
Important information about some ingredients of Riastap
Riastap contains up to 164 mg (7.1 mmol) of sodium per vial. This corresponds to 11.5 mg (0.5 mmol) of sodium per kg of body weight of the patient, if the recommended initial dose of 70 mg/kg body weight is administered. This should be taken into account when using it in patients on a controlled sodium diet.
3. How to use Riastap
Therapy should be started and supervised by a doctor experienced in the treatment of this type of disease.
Dosage
The required amount of human fibrinogen and the duration of therapy depend on:
- the severity of the disease
- the location and intensity of bleeding
- the patient's clinical condition. Using a higher dose of Riastap than recommendedThe doctor should regularly check the blood clotting status during therapy. In case of overdose, the risk of thromboembolic complications is increased.
Method of administration
In case of questions about the use of this product, you should consult a doctor or pharmacist (see the section "Information intended exclusively for healthcare professionals").
4. Possible side effects
Like all medicines, Riastap can cause side effects, although they may not occur in everyone.
You should contact your doctor immediately:
- in case of any side effects
- in case of side effects not listed in this leaflet.
The following adverse reactions have been observed very commonly(may occur in more than 1 in 10 people):
- Body temperature increase
The following adverse reactions have been observed uncommonly(in less than 1 in 100 people):
- Sudden allergic reaction (such as skin redness, skin rash all over the body, blood pressure drop, breathing difficulties).
The following adverse reactions have been observed commonly(in less than 1 in 10 people, although these incidents occur more frequently in patients not receiving fibrinogen):
- Increased risk of blood clot formation (see section 2. "Warnings and precautions").
Reporting side effects
If any side effects occur, including any side effects not listed in the leaflet, the doctor or pharmacist should be informed. Side effects can be reported directly to the Department of Monitoring of Adverse Reactions to Medicinal Products of the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products
Al. Jerozolimskie 181C
02-222 Warsaw
tel.: +48 22 49 21 301
fax: +48 22 49 21 309
Website: ://smz.ezdrowie.gov.pl
Side effects can also be reported to the marketing authorization holder.
By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Riastap
- Store the medicine out of sight and reach of children.
- Do not use Riastap after the expiry date stated on the label and carton.
- Do not store above 25°C. Do not freeze.
- To protect from light, the vial should be stored in the outer packaging.
- The reconstituted solution should be used immediately.
- If the prepared solution is not administered immediately, its storage time should not exceed 8 hours at room temperature (max. 25 ºC).
- The reconstituted solution should not be stored in the refrigerator.
6. Contents of the pack and other information
What Riastap contains The active substance is:
Human fibrinogen (1 g/vial; after reconstitution in 50 ml of water for injection, approximately 20 mg/ml).
To obtain more detailed information, see the section "Information intended exclusively for healthcare professionals".
Other ingredients are:
Human albumin, sodium chloride, L-arginine hydrochloride, sodium citrate, sodium hydroxide (for pH adjustment),
See the last section of section 2. "Important information about some ingredients of Riastap".
What Riastap looks like and what the pack contains
Riastap is a white powder.
After reconstitution in water for injection, the solution should be clear or slightly opalescent, i.e., it may shimmer when viewed in light but should not contain any visible particles.
Packaging
Packaging containing 1 g (Fig. 1)
One vial of 1 g of human fibrinogen
Filter: Pall syringe filter
Dosing spike: Mini-Spike dosing spike

Fig.1
Marketing Authorization Holder and Manufacturer:
CSL Behring GmbH
Emil-von-Behring-Str. 76
35041 Marburg
Germany
This medicinal product is registered in the EEA Member States under the following names:
Riastap 1g, powder for solution for injection/infusion
United Kingdom
Riastap 1g, poudre pour solution injectable/perfusion
France
Riastap 1g, prašek za raztopino za injiciranje ali infundiranje
Slovenia
Riastap 1g
Germany, Ireland
Riastap
Belgium, Cyprus, Denmark, Finland, Greece, Iceland, Italy, Luxembourg, Malta, Norway, Poland, Slovakia, Spain, Sweden
Date of approval of the leaflet: September 2024
Information intended exclusively for healthcare professionals Dosage:
The fibrinogen level (functional) should be determined to calculate the individual dosage and the amount and frequency of doses administered to each patient through regular measurements of fibrinogen levels in plasma and continuous monitoring of the patient's clinical condition, taking into account other replacement therapies used.
Normal fibrinogen levels in plasma are between 1.5 and 4.5 g/l. The critical fibrinogen level in plasma, below which bleeding may occur, is approximately 0.5-1 g/l. In the case of major surgical procedures, careful monitoring of replacement therapy with coagulation tests is necessary.
Initial dose
If the patient's fibrinogen level is not known, the recommended dose administered intravenously is 70 mg per kg of body weight.
Subsequent dose
The target level (1 g/l) in cases of minor bleeding (e.g., nosebleeds, muscle bleeding, or menstrual bleeding) should be maintained for at least three days. The target level (1.5 g/l) in cases of major bleeding (e.g., head trauma or intracranial bleeding) should be maintained for seven days.
Fibrinogen dose =
[Target level (g/l) - measured level (g/l)]
(mg/kg body weight)
0.017 (g/l per mg/kg body weight)
Dosage in newborns, infants, and children
There are limited data from clinical trials on the dosing of Riastap in children.
The recommendations for dosing in children are the same as in adults, based on both these studies and long-term clinical experience with fibrinogen preparations.
Method of administration
General information
- The reconstitution and withdrawal process should be carried out under aseptic conditions.
- Reconstituted products should be visually inspected before administration to exclude the presence of particles and discoloration.
- The solution should be almost colorless to yellowish, clear to slightly opalescent, with a neutral pH. Do not use solutions that are cloudy or contain sediment.
Reconstitution
- Both the solvent and the powder should be warmed to room temperature or body temperature (not above 37°C) without opening the vials.
- Riastap should be reconstituted with water for injection (50 ml, not included).
- Before reconstituting the product, hands should be washed or gloves should be put on.
- The cap should be removed from the vial containing Riastap, exposing the central part of the infusion stoppers.
- The surface of the infusion stopper should be wiped with an antiseptic solution and left to dry.
- The diluent should be introduced into the vial using suitable equipment. Ensure that the powder is completely moistened.
- After gentle mixing by rotating the vial until the powder is reconstituted, the solution is ready for administration. Avoid vigorous shaking, which can cause foaming. It is generally accepted that the powder dissolves within about 5 minutes. Complete dissolution should not take longer than 15 minutes.
- Open the plastic blister containing the dosing spike (Mini-Spike) provided with the Riastap medicinal product (Fig. 2).

Fig. 2
- The provided dosing spike should be inserted into the stopper of the vial with the reconstituted product (Fig. 3)

Fig. 3
- After inserting the dosing spike, the cap should be removed. After removing the cap, the exposed surface should not be touched.
- Open the blister pack with the filter (Pall syringe filter) provided with the Riastap medicinal product (Fig. 4)

Fig. 4
Screw the syringe onto the filter (Fig. 5)
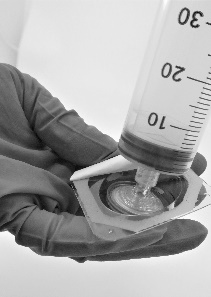
Fig. 5
- Screw the syringe with the attached filter onto the dosing end (Fig. 6)

Fig. 6
- Withdraw the reconstituted product into the syringe (Fig. 7)
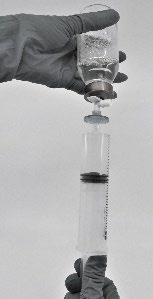
Fig.7
- After completion, the filter, dosing spike, and empty vial should be disconnected from the syringe and disposed of according to local regulations. Then, start administering the medicine according to the recommendations. The reconstituted solution should be administered immediately using a separate injection/infusion set.
- Be careful not to cause blood aspiration into syringes filled with the product.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
Method of administration
For intravenous administration of the reconstituted solution at room temperature, a standard infusion set is recommended. The reconstituted solution should be injected or infused slowly, at a rate suitable for the patient's comfort.
The rate of injection or infusion should not exceed approximately 5 ml per minute.
- Country of registration
- Active substance
- Prescription requiredNo
- Manufacturer
- ImporterCSL Behring GmbH
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to RiastapDosage form: Powder, 1 gActive substance: fibrinogen, humanPrescription not requiredDosage form: Powder, 1000 IUActive substance: coagulation factor VIIIManufacturer: CSL Behring GmbHPrescription requiredDosage form: Powder, 2000 IUActive substance: coagulation factor VIIIManufacturer: CSL Behring GmbHPrescription required
Alternatives to Riastap in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Riastap in Ukraine
Alternative to Riastap in Spain
Online doctors for Riastap
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Riastap – subject to medical assessment and local rules.














