How to use Prepidil
Package Leaflet: Information for the User
Warning! Keep the leaflet. Information on the immediate packaging in a foreign language.
Prepidil
500 μg/3 g, cervical gel
Dinoprostone
You should read the leaflet carefully before using the medicine, as it contains important information for the patient.
- You should keep this leaflet, so you can read it again if you need to.
- You should consult a doctor or pharmacist if you have any further questions.
- This medicine has been prescribed specifically for you. Do not pass it on to others. It may harm them, even if their symptoms are the same as yours.
- If any of the side effects get serious, or if you notice any side effects not listed in this leaflet, please tell your doctor, pharmacist, or nurse. See section 4.
Table of Contents of the Leaflet:
- 1. What is Prepidil and what is it used for
- 2. Important information before using Prepidil
- 3. How to use Prepidil
- 4. Possible side effects
- 5. How to store Prepidil
- 6. Contents of the pack and other information
1. What is Prepidil and what is it used for
Prepidil is used to help induce labor at term when this is necessary for obstetric or medical reasons. Dinoprostone is a prostaglandin with a luteolytic effect. Prostaglandins can stimulate the contraction of smooth muscle in the uterus and modulate the response of these organs to hormonal stimuli.
2. Important information before using Prepidil
When not to use Prepidil:
- high-order multiple pregnancy (six or more reported pregnancies),
- delayed engagement of the fetal head,
- previous uterine surgery, such as cesarean section or hysterectomy,
- pelvic disproportion,
- acute obstetric conditions where the benefit-to-risk ratio for the fetus or mother favors surgical intervention,
- fetal presentation other than cephalic,
- clinical suspicion of fetal distress based on fetal heart rate monitoring,
- infection of the lower genital tract,
- undiagnosed vaginal discharge and/or abnormal bleeding during pregnancy,
- difficult and/or traumatic delivery in history,
- presenting part above the pelvic inlet,
- active heart, lung, kidney, or liver disease,
- placenta previa.
Warnings and precautions
Prepidil should only be used in hospitals and clinics with specialized obstetric units and only when 24-hour medical care is ensured. Before and during its administration, careful monitoring of uterine contractions, fetal status, and cervical characteristics (dilation, softening, and effacement) is necessary to detect any adverse reactions, such as excessive uterine stimulation or fetal distress. In cases of previously diagnosed uterine hypertonus or excessive uterine contractility, the doctor should recommend continuous monitoring of contractions and maternal and fetal status. Caution is required when administering Prepidil to patients with circulatory, liver, or kidney disorders, active asthma, glaucoma (or increased intraocular pressure), epilepsy, hypertension, or placental abruption. In cases of prolonged strong contractions, the risk of uterine rupture should always be considered. Before using Prepidil, the pelvic-gestational ratio should be carefully evaluated. Treatment of patients with excessive uterine tone or contractility, or those with concerns about fetal heart rate, should aim to maintain good maternal and fetal status. As with all substances with an effect similar to oxytocin, in cases of excessive uterine activity or abnormal uterine pain, the possibility of uterine rupture should be considered. Uterine rupture can lead to the introduction of fetal tissue into the maternal circulation, which can cause an anaphylactoid reaction. The use of exogenous prostaglandins can enhance the reaction to oxytocin. Prepidil should not be applied above the internal cervical os, as excessive uterine stimulation has been observed with extra-amniotic administration.
Other medicines and Prepidil
Prostaglandins enhance the effect of oxytocin on the uterus, so they should not be administered simultaneously. If these medicines are used sequentially, close monitoring of the patient is recommended. The recommended time interval between intravenous oxytocin administration and Prepidil use is 6 to 12 hours. You should tell your doctor about any alcohol consumption and all medicines you are currently taking or have recently taken, as well as any medicines you plan to take. You should consult your doctor about the possibility of using Prepidil with other medicines.
Using Prepidil in patients with kidney or liver disorders
Patients with severe kidney or liver disease associated with metabolic disorders should be closely monitored.
Prepidil with food and drink
You should inform your doctor about any alcohol consumption.
Pregnancy and breastfeeding
Prepidil is used only in pregnancy to help induce labor.
3. How to use Prepidil
Prepidil is intended for use only under the supervision of a doctor in hospitals and clinics equipped with facilities for monitoring the patient's and fetus's condition. The dose and frequency of administration are determined by the doctor. In case of doubts, you should consult a doctor. (See Information intended for healthcare professionals only).
4. Possible side effects
Like all medicines, Prepidil can cause side effects, although not everybody gets them. The frequency and severity of side effects of Prepidil are dose-dependent and also depend on the route of administration. During treatment with Prepidil, the following side effects have been observed:
In the mother:
- hypersensitivity reactions (e.g., anaphylactic reaction, anaphylactic shock, pseudo-anaphylactic reaction),
- abnormal uterine contractions (increased frequency, intensity, and duration) with or without changes in fetal heart rate,
- hypertension,
- cardiac arrest,
- uterine rupture,
- nausea, vomiting, and diarrhea,
- pulmonary embolism/amniotic fluid embolism,
- short wheezing sounds heard over the lungs,
- bronchospasm/asthma,
- shortness of breath,
- feeling of pressure in the chest,
- cough,
- back pain,
- placental abruption,
- sudden cervical dilation,
- fever,
- feeling of heat, pain, irritation in the vagina,
- rash. In patients, an increase in body temperature and white blood cell count may occur, which resolves after treatment is discontinued.
In the fetus/newborn:
- fetal distress/fetal heart rate abnormalities during or after treatment with Prepidil,
- premature birth,
- fetal death, stillbirth, neonatal death; especially after severe side effects, such as uterine rupture,
- neonatal assessment score less than 7 points on the Apgar scale,
- fetal acidosis.
As with any other intravaginally administered drug, the risk of local infections should be considered. If an infection occurs, appropriate treatment should be initiated. Other side effects not listed above may occur with the use of Prepidil.
Reporting side effects
If you experience any side effects, including those not listed in this leaflet, you should inform your doctor, pharmacist, or nurse. Side effects can be reported directly to the Department of Adverse Reaction Monitoring of Medicinal Products, Medical Devices, and Biocidal Products, Al. Jerozolimskie 181C, 02-222 Warsaw, Tel.: +48 22 49 21 301, Fax: +48 22 49 21 309, website: https://smz.ezdrowie.gov.pl. By reporting side effects, you can help provide more information on the safety of this medicine.
5. How to store Prepidil
Store in a refrigerator (2°C - 8°C). Keep out of the sight and reach of children. Do not use this medicine after the expiry date stated on the packaging. The expiry date refers to the last day of the month. Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
6. Contents of the pack and other information
What Prepidil contains
- The active substance is dinoprostone (dinoprostonum). 3 g of gel contains 500 micrograms of dinoprostone.
- The other ingredients are colloidal anhydrous silica and triacetin.
What Prepidil looks like and contents of the pack
3 g of gel in a polyethylene syringe placed in a sterile package and a catheter made of plastic material placed in a sterile package, in a cardboard box. For more detailed information, please contact the marketing authorization holder or parallel importer.
Marketing authorization holder in Croatia, the country of export:
Pfizer Croatia d.o.o., Slavonska avenija 6, 10000 Zagreb, Croatia
Manufacturer:
Pfizer Manufacturing België NV, Rijksweg 12, 2870 Puurs, Belgium
Parallel importer:
Delfarma Sp. z o.o., ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź
Repackaged by:
Delfarma Sp. z o.o., ul. Św. Teresy od Dzieciątka Jezus 111, 91-222 Łódź, Marketing authorization number in Croatia, the country of export: HR-H-599786838-01
Parallel import authorization number: 353/24
Date of leaflet approval: 03.10.2024
[Information about the trademark]
-------------------------------------------------------------------------------------------------------------------------
Information intended for healthcare professionals only:
To administer the medicine correctly, the patient should be in a supine position and have a vaginal speculum in place. The entire contents of the syringe (500 μg of dinoprostone = 3 g of Prepidil) should be injected in a sterile manner into the cervical canal using the catheter provided with the package. Prepidil should not be administered above the internal cervical os. After administration of the gel, the patient should remain in a supine position for at least 15 minutes to minimize gel leakage. The syringe contents are for single-patient use only. Do not attempt to administer the remaining gel in the catheter. Avoid skin contact with the medicine and wash your hands after application. After use, the syringe, catheter, and any unused packaging should be disposed of according to local regulations. INSTRUCTIONS FOR USE OF THE SYRINGE Remove the sterile catheter and syringe from the outer packaging.
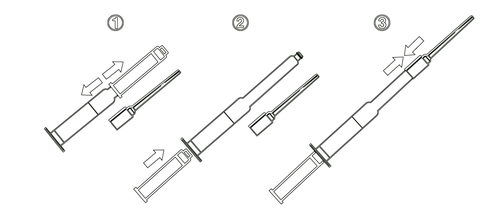
- 1. Remove the protective cap (the cap will serve as an extension of the plunger).
- 2. Insert the cap into the syringe.
- 3. Securely attach the free end of the sterile catheter to the end of the syringe. Proper attachment will be confirmed by a clicking sound. Then, start administering the gel.
In case of overdose of Prepidil
Prepidil is available only in single-dose packaging, so overdose symptoms can occur under normal circumstances only in patients with hypersensitivity to the medicine. Overdose symptoms may include: excessive uterine stimulation or abnormally intense or frequent uterine contractions, which can lead to fetal distress. In case of overdose, symptomatic treatment should be used. If prolonged excessive uterine stimulation and/or fetal distress occur after discontinuation of Prepidil, intravenous administration of a beta-mimetic may be beneficial. If symptomatic treatment is ineffective, immediate delivery is indicated.
Explanation of symbols on the catheter packaging
STERILE ENDOCERVICAL CATHETER – sterile catheter for the cervix

Medical device

Sterile single barrier system, sterilized by radiation

For single use

Do not sterilize again

Do not use if packaging is damaged

Expiration date of the catheter

Batch number of the catheter

Catheter manufacturer
- Country of registration
- Active substance
- Prescription requiredNo
- Marketing authorisation holder (MAH)Pfizer Croatia d.o.o.
- This information is for reference only and does not constitute medical advice. Always consult a licensed doctor before taking any medication. Oladoctor is not responsible for medical decisions based on this content.
- Alternatives to PrepidilDosage form: System, 10 mgActive substance: dinoprostoneManufacturer: Ferring GmbHPrescription not requiredDosage form: Gel, 0.5 mg/3 gActive substance: dinoprostonePrescription not requiredDosage form: Tablets, 25 mcgActive substance: misoprostolPrescription required
Alternatives to Prepidil in other countries
The best alternatives with the same active ingredient and therapeutic effect.
Alternative to Prepidil in Ukraine
Alternative to Prepidil in Spain
Online doctors for Prepidil
Discuss dosage, side effects, interactions, contraindications, and prescription renewal for Prepidil – subject to medical assessment and local rules.